All Photos(1)
About This Item
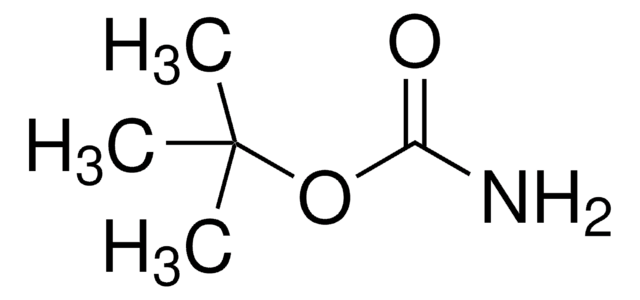
Linear Formula:
(CH3)3CN=C=NC(CH3)3
CAS Number:
Molecular Weight:
154.25
Beilstein:
1758049
EC Number:
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
reaction suitability
reaction type: Coupling Reactions
refractive index
n20/D 1.428 (lit.)
bp
48-50 °C/12 mmHg (lit.)
density
0.8 g/mL at 25 °C (lit.)
application(s)
peptide synthesis
functional group
amine
SMILES string
CC(C)(C)N=C=NC(C)(C)C
InChI
1S/C9H18N2/c1-8(2,3)10-7-11-9(4,5)6/h1-6H3
InChI key
IDVWLLCLTVBSCS-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Application
N,N′-Di-tert-butylcarbodiimide can be used:
- As a reagent for the guanylation of aryl amines catalyzed by lanthanum amides.
- To prepare dichloroimidazolidine-4,5-dione by reacting with oxalyl chloride, which is a key intermediate for the synthesis of N,N′-diamidocarbenes.
- To prepare the iridium complex of benzamidine named Ir(FMeppy)2(N,N′-di-tert-butyl-4-methyl-benzamidine).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
95.0 °F - closed cup
Flash Point(C)
35 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Qinghai Li et al.
The Journal of organic chemistry, 72(18), 6763-6767 (2007-08-07)
It is demonstrated that the cyclopentadienyl-free simple lanthanide amides [(Me(3)Si)(2)N](3)Ln(mu-Cl)Li(THF)(3)(Ln = La, Sm, Eu, Y, Yb) and Ln[N(SiMe(3))(2)]3 (Ln = Y, Yb) are highly efficient catalysts for the guanylation of both aromatic and secondary amines with a high activity under
Synthesis, characterization and photophysical properties of iridium complexes with amidinate ligands
Sahin C, et al.
Journal of Organometallic Chemistry, 772, 68-78 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service