All Photos(2)
About This Item
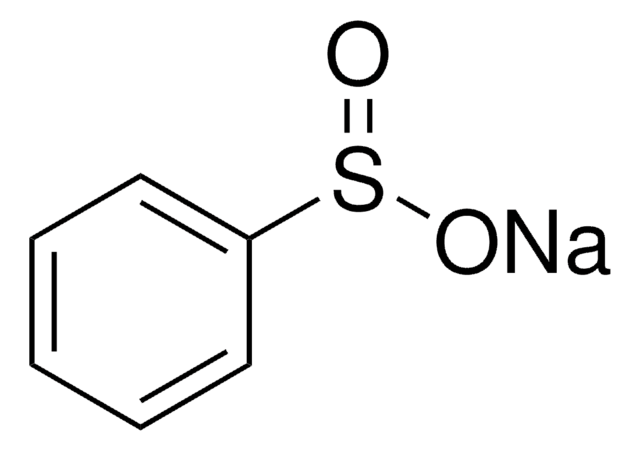
Linear Formula:
C6H5SeO2H
CAS Number:
Molecular Weight:
189.07
Beilstein:
1929765
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
121-124 °C (lit.)
SMILES string
O[Se](=O)c1ccccc1
InChI
1S/C6H6O2Se/c7-9(8)6-4-2-1-3-5-6/h1-5H,(H,7,8)
InChI key
WIHKGDVGLJJAMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzeneseleninic acid oxidizes hydrazine or 1,2-disubstituted derivatives to corresponding diazenes.
Application
Benzeneseleninic acid was used as catalyst during the oxidation of sulfides to sulfoxides via ligand coupling on the iodine atom. It was also used in the preparation of 3-methoxy-17a-oxa-D-homoestra-l,3,5(10)-trien-17-one.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oxidation of hydrazines with benzeneseleninic acid and anhydride.
Back TG, et al.
The Journal of Organic Chemistry, 46(8), 1564-1570 (1981)
Facile oxidation of sulfides to sulfoxides using iodosobenzene and benzeneseleninic acid as a catalyst.
Roh KR, et al.
Tetrahedron Letters, 32(6), 793-796 (1991)
1, 2-Hydrogen shifts in thermal and photic Bamford-Stevens reactions of cyclohexanones. Activation by an endocyclic oxygen.
Olmstead KK and Nickon A.
Tetrahedron, 54(40), 12161-12172 (1998)
S Zheng et al.
Hua xi yi ke da xue xue bao = Journal of West China University of Medical Sciences = Huaxi yike daxue xuebao, 26(4), 371-374 (1995-12-01)
In view of the negative relationship between selenium and cancer, we designed and synthesized eleven substituted benzeneselenic acids (IVa-k) The antineoplastic activity of the title compounds was evaluated via the tests of inhibition of acetylcholinesterase, HL-60 and K562 in vitro
Craig A Bayse
Journal of inorganic biochemistry, 104(1), 1-8 (2009-10-30)
The toxicity of selenium is a major barrier to its application to the prevention of cancer, cardiovascular disease, and other chronic ailments. Organic seleninic acids, as well as other reducible selenium compounds, have been shown to react with biological sulfhydryls
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service