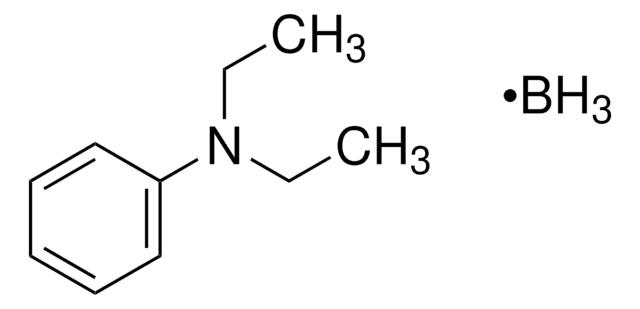
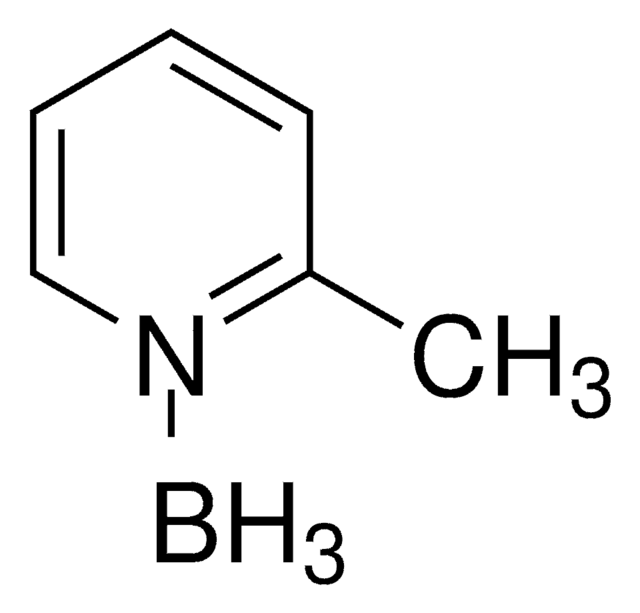
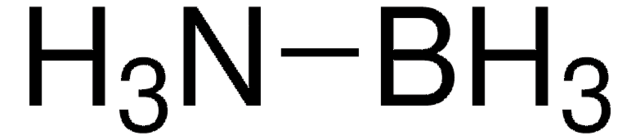178977
Borane triethylamine complex
97%
Synonym(s):
(N,N-Diethylethanamine)trihydroboron, NSC 59740, Triethylamine borane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)3N · BH3
CAS Number:
Molecular Weight:
115.02
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
reaction suitability
reagent type: reductant
refractive index
n20/D 1.442 (lit.)
bp
97 °C/12 mmHg (lit.)
mp
−4 °C (lit.)
density
0.777 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
[BH3-][N+](CC)(CC)CC
InChI
1S/C6H18BN/c1-4-8(7,5-2)6-3/h4-6H2,1-3,7H3
InChI key
ONRDAGWFOUZNLV-UHFFFAOYSA-N
Application
Borane triethylamine complex is a general reagent which can be used in the synthesis of silylhydroboranes and meso-triarylsubporphyrins. It can also be used in the preparation of liquid-phase hydrogen storage material.
Used for:
Reactant for the synthesis of:
Reactant for modification of the radionuclide 211 At for astatination of biomolecules
- Ethanolysis of amine-borane adducts producing a reducing system
- Photochemical hydroboration and oxidation of single-walled carbon nanotubes
Reactant for the synthesis of:
- Silylboranate
- N-heterocyclic carbene borane complexes via Lewis base exchange with amine-boranes
- Boron carbide nitride films via low-pressure CVD
- (Pyridine)tripyrrolylboron
Reactant for modification of the radionuclide 211 At for astatination of biomolecules
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
19.4 °F - closed cup
Flash Point(C)
-7 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
ABC?Type meso?Triaryl?Substituted Subporphyrins.
Yoshida K, et al.
European Journal of Organic Chemistry, 2014(19), 3997-4004 (2014)
Rational Synthesis of A2B-type meso-Triarylsubporphyrins.
Tanaka T, et al.
Organic Letters, 14(11), 2694-2697 (2012)
The quest for silylhydroboranes:(Me3Si)3SiBH2.
Arp H, et al.
Dalton Transactions, 39(39), 9270-9274 (2010)
A single-component liquid-phase hydrogen storage material.
Luo W, et al.
Journal of the American Chemical Society, 133(48), 19326-19329 (2011)
Zizwe A Chase et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(46), 16541-16546 (2015-09-26)
The state of Ni supported on HZSM-5 zeolite, silica, and sulfonated carbon was studied during aqueous-phase catalysis of phenol hydrodeoxygenation using in situ extended X-ray absorption fine structure spectroscopy. On sulfonated carbon and HZSM-5 supports, NiO and Ni(OH)2 were readily
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service