147400
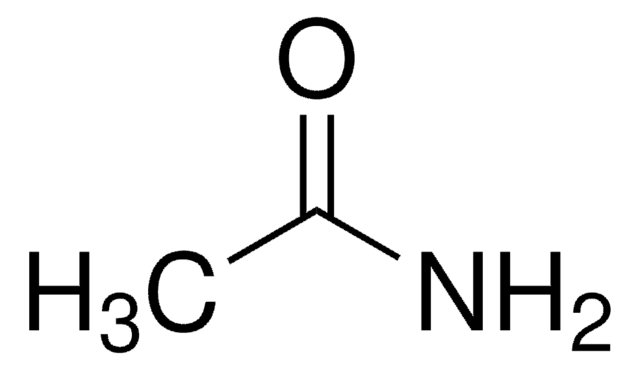
N-Ethylacetamide
99%
Synonym(s):
Acetamidoethane, N-Acetylethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
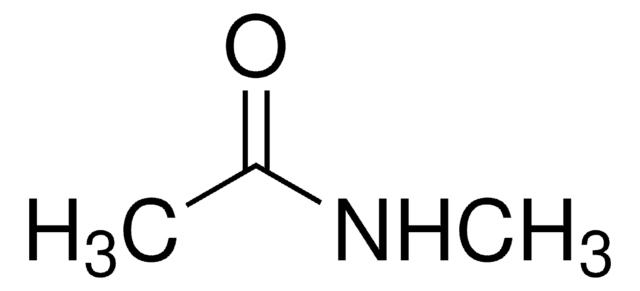
CH3CONHC2H5
CAS Number:
Molecular Weight:
87.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.433 (lit.)
bp
90-92 °C/8 mmHg (lit.)
density
0.924 g/mL at 25 °C (lit.)
SMILES string
CCNC(C)=O
InChI
1S/C4H9NO/c1-3-5-4(2)6/h3H2,1-2H3,(H,5,6)
InChI key
PMDCZENCAXMSOU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Ethylacetamide is a suitable nylon model for mechanistic studies of dye fading.
Application
N-Ethylacetamide was used to investigate propylene carbonate and a mixture of N-methylformamide and N-ethylacetamide by broadband dielectric and mechanical shear spectroscopy. It was used in determination of reaction products of OH-oxidation of N-methylpyrrolidone under atmospheric conditions.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jolanta Świergiel et al.
Physical chemistry chemical physics : PCCP, 13(9), 3911-3916 (2011-01-07)
The impedance spectroscopy studies performed for two strongly hydrogen-bonded liquid amides: N-methylpropionamide (NMP, CH(3)·NH·CO·C(2)H(5)) and N-ethylacetamide (NEA, C(2)H(5)·NH·CO·CH(3)) have shown that the two centers of the peptide linkage, -NH·CO-, active in the C=OH-N hydrogen bonds formation, exhibit quite different sensibilities
Catalin Gainaru et al.
The Journal of chemical physics, 137(6), 064508-064508 (2012-08-18)
Propylene carbonate and a mixture of two secondary amides, N-methylformamide and N-ethylacetamide, are investigated by means of broadband dielectric and mechanical shear spectroscopy. The similarities between the rheological and the dielectric responses of these liquids and of the previously investigated
Tetsuya Tatsukawa et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 26(18), 4820-4825 (2006-05-05)
AMPA receptor (AMPAR) internalization provides a mechanism for long-term depression (LTD) in both hippocampal pyramidal neurons and cerebellar Purkinje cells (PCs). Cerebellar LTD at the parallel fiber (PF)-PC synapse is the underlying basis of motor learning and requires AMPAR activation
Li-Min Wang et al.
The Journal of chemical physics, 123(5), 054516-054516 (2005-08-20)
Dielectric relaxation dynamics of secondary amides is explored in their supercooled state near the glass transition temperature Tg by investigating N-ethylacetamide and its mixtures with N-methylformamide. All the samples are found to exhibit giant dielectric permittivities, reaching over 500 in
R Buchet et al.
Biophysical chemistry, 22(4), 249-254 (1985-10-01)
It is shown that a striking parallelism exists between the anesthetic potency of general halocarbon anesthetics and their influence on the hydrogen bond association constants in N-H...O=C type hydrogen bonds, important for shaping the ion channels. It is further shown
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service