145947
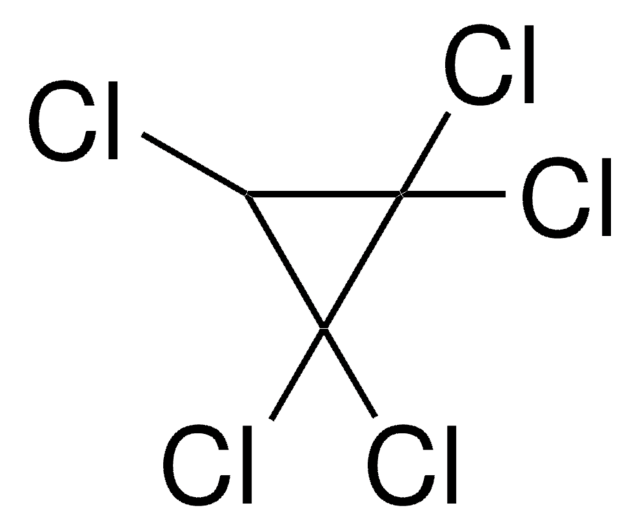
Tetrachlorocyclopropene
98%
Synonym(s):
1,2,3,3-Tetrachlorocyclopropene, Perchlorocyclopropene, Tetrachlorocyclopropenone, Tetrachlorocycloproprene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3Cl4
CAS Number:
Molecular Weight:
177.84
Beilstein:
1099206
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.507 (lit.)
bp
125-130 °C (lit.)
density
1.45 g/mL at 25 °C (lit.)
functional group
chloro
storage temp.
2-8°C
SMILES string
ClC1=C(Cl)C1(Cl)Cl
InChI
1S/C3Cl4/c4-1-2(5)3(1,6)7
InChI key
BLZOHTXDDOAASQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetrachlorocyclopropene undergoes electrochemical reductive tert-butyldimethylsilylation to yield 1,2,3-tris(tert-butyldimethylsilyl)cyclopropene. It undergoes alkylation reaction with ferrocene in dichloromethane and AlCl3 to yield 2,3-diferrocenylcyclopropenone.
Application
Tetrachlorocyclopropene was used to prepare starting reagent for synthesis of 3,3-diethyl-and 3,3-dibenzyl-1,2-diferrocenylcyclopropenes.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Elena I Klimova et al.
Organic & biomolecular chemistry, 1(24), 4458-4464 (2004-01-20)
Reactions of 2,3-diferrocenylcyclopropenone 1 with ethyl- and benzylmagnesium chlorides afford 3,3-diethyl-and 3,3-dibenzyl- 1,2-diferrocenylcyclopropenes 2 and 3, respectively, and products of nucleophilic opening of the three-membered ring resulting from the addition of RMgCl to the carbonyl group, viz., saturated ketones(4,5-diferrocenylheptan-3-ones 4a,b
H A Buchholz et al.
Proceedings of the National Academy of Sciences of the United States of America, 96(18), 10003-10005 (1999-09-01)
Electrochemical reductive tert-butyldimethylsilylation of tetrachlorocyclopropene to 1,2,3-tris(tert-butyldimethylsilyl)cyclopropene, a potential strained precursor for Diels-Alder and related cycloaddition reactions, is described. By hydride abstraction with nitrosonium tetrafluoroborate, 1,2,3-tris(tert-butyldimethylsilyl)cyclopropene is ionized quantitatively to Hückeloid 2pi aromatic tris(tert-butyldimethylsilyl)cyclopropenium tetrafluoroborate.
Jesús García-Valdés et al.
Journal of inorganic biochemistry, 197, 110689-110689 (2019-05-18)
Bis-cations with two 2,3-diferrocenylcyclopropenium fragments 3a-d, and the cis-2-(1,2-diferrocenylvinyl)-2-imidazolinium tetrafluoroborates 4a, d or the cis-2-(1,2-diferrocenylvinyl)-3,4,5,6-tetrahydropyrimidin-2-ium tetrafluoroborates 4b, c were obtained by interactions of 2,3-diferrocenyl-1-ethoxycyclopropenium tetrafluoroborate 1 with bis-1,4-N,N-(2a, d) or bis-1,5-N,N-(2b, c) nucleophiles. The reactions of 3a-d with sodium azide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service