128422
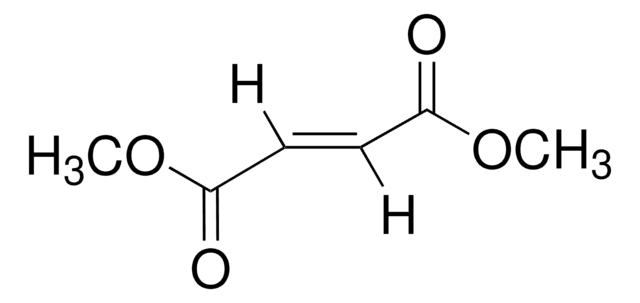
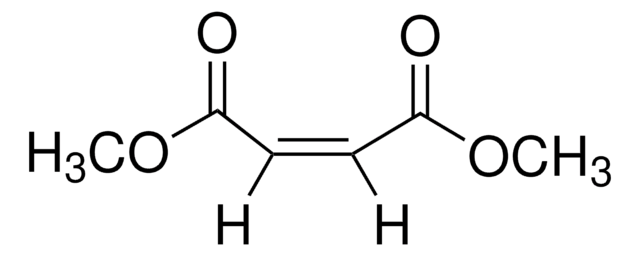
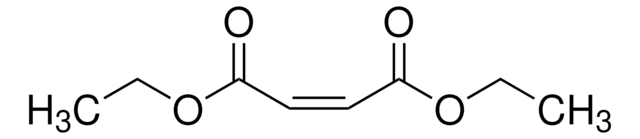
mono-Ethyl fumarate
95%
Synonym(s):
Fumaric acid monoethyl ester, Monoethyl fumarate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OCOCH=CHCOOH
CAS Number:
Molecular Weight:
144.13
Beilstein:
1723588
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
bp
147 °C/16 mmHg (lit.)
mp
66-68 °C (lit.)
SMILES string
CCOC(=O)\C=C\C(O)=O
InChI
1S/C6H8O4/c1-2-10-6(9)4-3-5(7)8/h3-4H,2H2,1H3,(H,7,8)/b4-3+
InChI key
XLYMOEINVGRTEX-ONEGZZNKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
mono-Ethyl fumarate (fumaric acid monoethyl ester, monoethyl fumarate) was used in the preparation of photo-crosslinkable macromers. It was also used to synthesize Ugi/intramolecular Diels-Alder (IMDA) cycloaddition products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Paulvannan
The Journal of organic chemistry, 69(4), 1207-1214 (2004-02-14)
An efficient approach to rigid tricyclic nitrogen heterocycles via sequential and tandem Ugi/intramolecular Diels-Alder (IMDA) cycloaddition of pyrrole is described. The one-pot Ugi four-component condensation (4CC) reaction was used as the key transformation to prepare trienes with a carboxamide substituent
Treatment of recurrent aphthous stomatitis with fumaric acid esters.
Emmanuella Guenova et al.
Archives of dermatology, 147(3), 282-284 (2011-03-23)
Janine Jansen et al.
Biomacromolecules, 10(2), 214-220 (2008-12-19)
Polymer networks were prepared by photocross-linking fumaric acid monoethyl ester (FAME) functionalized, three-armed poly(D,L-lactide) oligomers using N-vinyl-2-pyrrolidone (NVP) as diluent and comonomer. The use of NVP together with FAME-functionalized oligomers resulted in copolymerization at high rates, and networks with gel
H B Thio et al.
The British journal of dermatology, 131(6), 856-861 (1994-12-01)
Systemic administration of fumaric acid (FA) derivatives was originally an empirical antipsoriatic treatment, which showed promising clinical results. In the present study, FURA-2-loaded suspensions of cultured normal keratinocytes and SV40-transformed keratinocytes (SVK-14 cells) were used to study the effects of
D Werdenberg et al.
Biopharmaceutics & drug disposition, 24(6), 259-273 (2003-09-16)
Psoriasis is a chronic inflammatory skin disease. Its treatment is based on the inhibition of proliferation of epidermal cells and interference in the inflammatory process. A new systemic antipsoriasis drug, which consists of dimethylfumarate and ethylhydrogenfumarate in the form of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service