All Photos(1)
About This Item
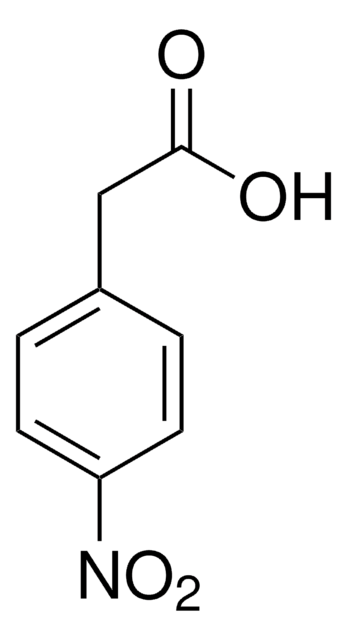
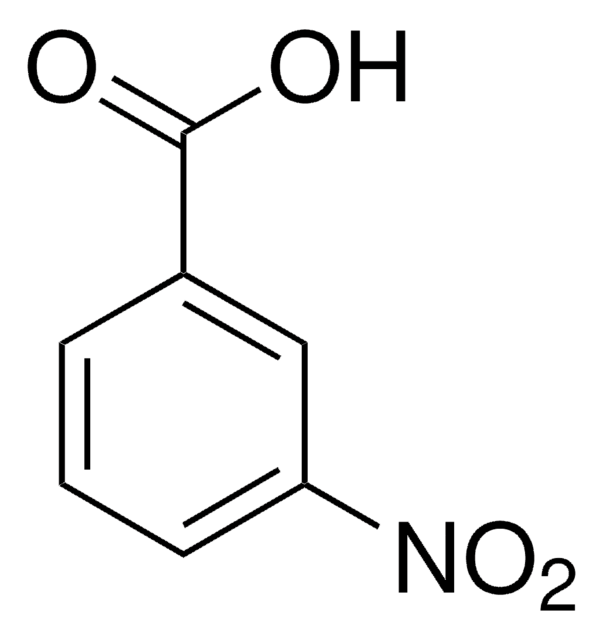
Linear Formula:
O2NC6H4CH2CO2H
CAS Number:
Molecular Weight:
181.15
Beilstein:
2050088
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
117-120 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
OC(=O)Cc1cccc(c1)[N+]([O-])=O
InChI
1S/C8H7NO4/c10-8(11)5-6-2-1-3-7(4-6)9(12)13/h1-4H,5H2,(H,10,11)
InChI key
WUKHOVCMWXMOOA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
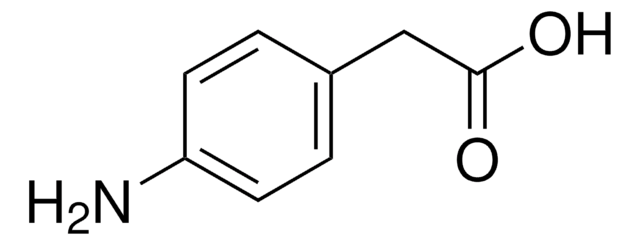
General description
3-Nitrophenylacetic acid is obtained by mild oxidation of 4-amino phenylacetic acid using potassium peroxymonosulfate as oxidizing agent in acetone.
Application
3-Nitrophenylacetic acid was used to study photodecarboxylation of nitrophenylacetate ions in aqueous solution. 3-Nitrophenylacetic acid was used in study to synthesize an azobenzene amino acid, having potent use as photo-inducible conformational switch in polypeptides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A mild oxidation of aromatic amines.
Webb KS and Seneviratne S.
Tetrahedron Letters, 36(14), 2377-2378 (1995)
Photodecarboxylation of nitrophenylacetate ions.
Margerum JD and Petrusis CT.
Journal of the American Chemical Society, 91(10), 2467-2472 (1969)
Andreas Aemissegger et al.
Nature protocols, 2(1), 161-167 (2007-04-03)
The synthesis of an azobenzene amino acid (aa) for use as a photo-inducible conformational switch in polypeptides is described. The compound can be easily incorporated into an aa sequence by solid-phase peptide synthesis using standard 9-fluorenylmethoxycarbonyl methods. A reversible conformational
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service