103543
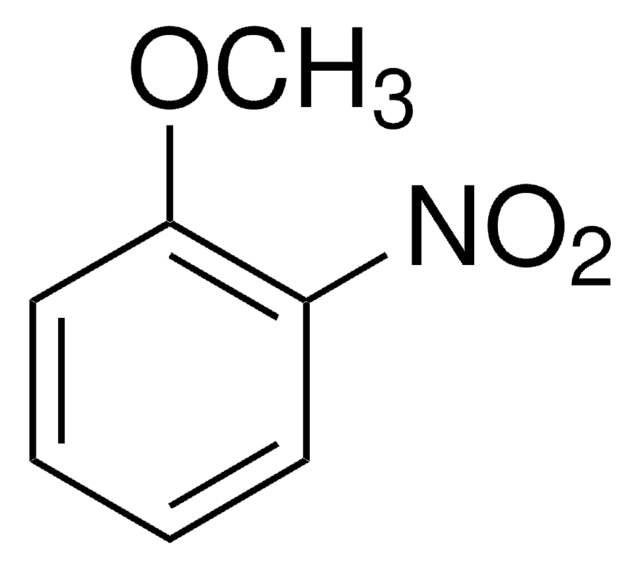
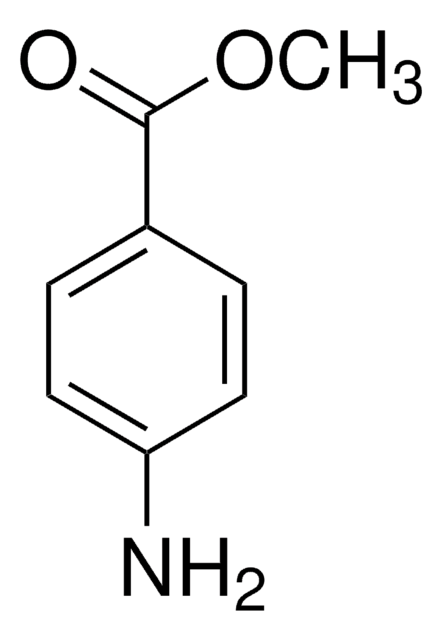
4-Nitroanisole
97%
Synonym(s):
1-Methoxy-4-nitrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4OCH3
CAS Number:
Molecular Weight:
153.14
Beilstein:
1865361
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39032065
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
density
1.233 g/mL at 25 °C (lit.)
functional group
nitro
SMILES string
COc1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H7NO3/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3
InChI key
BNUHAJGCKIQFGE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Nitroanisole undergoes photochemical nucleophilic aromatic substitution by hydroxide ion to form 4-methoxyphenol and 4-nitrophenol.
Application
4-Nitroanisole was used as probe to determine Π* of Kamlet-Taft solvent parameters and high pressure and supercritical water in the temperature range of 16-420 °C. 4-Nitroanisole was used as carbon and energy supplement for the isolation of Rhodococcus strains.
Biochem/physiol Actions
4-Nitroanisole is O-demethylated to 4-nitrophenol by human liver microsomes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Carc. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
266.0 °F - closed cup
Flash Point(C)
130 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Petr Klán et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 1(12), 1012-1016 (2003-03-29)
A temperature-sensitive photochemical nucleophilic aromatic substitution on 4-nitroanisole by a hydroxide ion in homogeneous solutions, in a two-phase system under phase-transfer catalysis conditions, and in the microwave field is reported. It was found that reaction regioselectivity dramatically changes with temperature
Song Chen et al.
Journal of economic entomology, 98(3), 943-946 (2005-07-19)
Cytochrome P450 monooxygenases are a major metabolic mechanism responsible for pyrethroid resistance in Helicoverpa armigera (Hübner) from Asia. Cytochrome P450-mediated O-demethylation activity toward p-nitroanisole (PNOD) of individual fourth instars was determined in five strains of H. armigera by using a
M S Romero-Cano et al.
Journal of controlled release : official journal of the Controlled Release Society, 82(1), 127-135 (2002-07-11)
The controlled release of 4-nitroanisole from polylactide nanoparticles with different morphologies is reported. Two theoretical equations have been used in an attempt to fit the experimental results. Good agreement between theory and experiment was found for short release time. The
V V Shumiantseva et al.
Voprosy meditsinskoi khimii, 44(4), 369-375 (1998-12-10)
Semisynthetic flavocytochromes, obtained by covalent binding of riboflavins with cytochrome P450 2B4, were able to catalyse H2O2-supported aniline p-hydroxylation, amidopyrine N-demethylation and p-nitroanisole O-dealkylation. Rates of these reactions were considerably higher than the rates of corresponding NAD(P)H-dependent reactions and comparable
H V Gelboin et al.
Biochemical pharmacology, 50(11), 1841-1850 (1995-11-27)
Cytochromes P450 3A3/4 are inordinately important P450 enzymes catalyzing the metabolism of a large variety of clinically useful drugs, steroids, and carcinogens. Two monoclonal antibodies, MAb 3-29-9 and MAb 275-1-2, were prepared to human P450 3A4 from mice immunized with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service