28564-U
SLB®-5ms Capillary GC Column
L × I.D. 20 m × 0.18 mm, df 0.18 μm
Synonym(s):
GC column, SPB-5, 5% diphenyl, mass spec
About This Item
Recommended Products
material
fused silica
Quality Level
Agency
Standard Method 6040D
EN 2005/108/EC
EPA 610,625,8015,8082,8100,8141,8270,OLM04.2 SVOA
EPA TO-13,IP-8,8270,525.2,608.1/608.2,608/8081/OLM04.2 PEST
JMHLW
NIOSH 2530,5503
OSHA 62
meets requirements for USP G27 and G36
suitable for EPA 1613
reg. compliance
FDA LIB 4423
parameter
-60-340 °C temperature (isothermal)
-60-360 °C temperature (programmed)
Beta value
250
df
0.18 μm
technique(s)
GC/MS: suitable
gas chromatography (GC): suitable (fast GC)
L × I.D.
20 m × 0.18 mm
matrix active group
Bonded and highly crosslinked; silphenylene polymer virtually equivalent in polarity to poly(5% diphenyl/95% dimethyl siloxane) phase
application(s)
agriculture
chemicals and industrial polymers
cleaning products
clinical
cosmetics
environmental
flavors and fragrances
food and beverages
forensics and toxicology
industrial hygiene
life science and biopharma
personal care
petroleum
pharmaceutical (small molecule)
column type
capillary non-polar
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
USP Code: This column meets USP G27 and G36 requirements.
Phase:
- Bonded and highly crosslinked
- Silphenylene polymer virtually equivalent in polarity to poly(5% diphenyl/95% dimethyl siloxane)
- ≤0.32 mm I.D.: -60 °C to 340 °C (isothermal) or 360 °C (programmed)
- ≥0.53 mm I.D.: -60 °C to 330 °C (isothermal) or 340 °C (programmed)
Other Notes
Legal Information
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Derivatization and Analysis of Amino Acids by GC-MS
Supel™ QuE Verde combines a novel carbon with zirconia coated silica (Z-Sep+) to provide an optimum balance between planar pesticide recovery and color removal.
Analysis of Tetrahydrocannabinol (THC) and Carboxytetrahydrocannabinol (THCCOOH) in Surface Waters by SPME and GC/MS
Sigma-Aldrich presents an article about the analysis of melamine and related compounds in dog food with the use of more economical gas chromatography-mass spectrometry (GC-MS) instrumentation.
Protocols
The proposed method appears to be reliable and sensitive for the determination of allergens in perfumes, following the SCCNFP Opinion.
Polychlorinated biphenyls (PCBs) were once widely used in many industrial and commercial applications such as insulating fluids, dielectrics, and lubricants. This included their extensive use in electrical transformers. PCB use has been banned in many countries, and the Stockholm Convention currently prohibits their production and use.
There are three types of analyses: GRO, DRO, and TPH. The published methods are simply variations of these to meet the different analytical needs of the individual agencies.
Separation of Cholesterol; Brassicasterol; Campesterol; Stigmasterol; β-Sitosterol
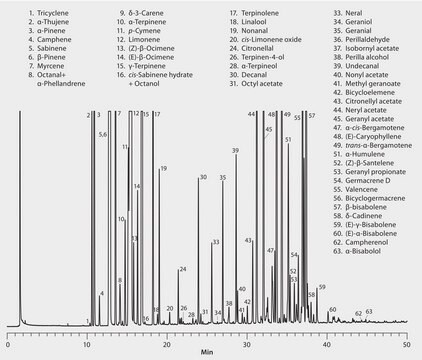
Chromatograms
suitable for GCsuitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service