T8658
Trypsin from bovine pancreas
suitable for protein sequencing, lyophilized powder
Synonym(s):
Porcine Trypsin, Trypsin for Mass Spectropetry
About This Item
Recommended Products
grade
Proteomics Grade
form
lyophilized powder
mol wt
23.8 kDa
packaging
vial of 100 μg
solubility
hydrochloric acid: soluble 1 mM, clear
suitability
suitable for protein sequencing
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Trypsin from bovine pancreas has been used for in-gel digestion for MS (mass spectrometry) analysis.
- It has been used for the digestion of albumin for size-exclusion chromatography.
- It has been used for the digestion of HDL (high density lipoprotein) for LC-MS (liquid chromatography-mass spectrometry) analysis.
- It has been used for limited proteolysis of IST1 (putative MAPK-activating protein PM28).
Biochem/physiol Actions
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Components
Caution
Unit Definition
Preparation Note
inhibitor
substrate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
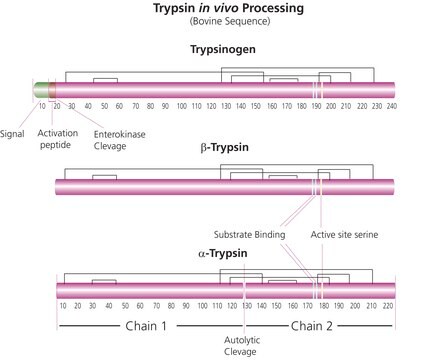
Trypsin is an enzyme in the serine protease class that consists of a polypeptide chain of 223 amino acid residues. Multiple sources, grades and formulations of trypsin specifically designed for research applications are available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service