All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C16H16N4O
CAS Number:
Molecular Weight:
280.32
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
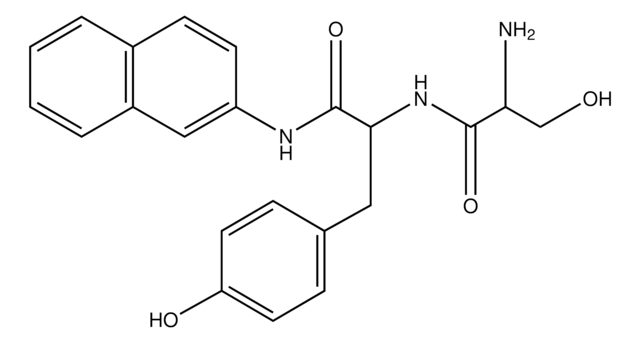
L-Histidine β-naphthylamide, powder
Assay
≥98% (TLC)
form
powder
color
white to faint yellow
storage temp.
−20°C
SMILES string
N[C@@H](Cc1cnc[nH]1)C(=O)Nc2ccc3ccccc3c2
InChI
1S/C16H16N4O/c17-15(8-14-9-18-10-19-14)16(21)20-13-6-5-11-3-1-2-4-12(11)7-13/h1-7,9-10,15H,8,17H2,(H,18,19)(H,20,21)/t15-/m0/s1
InChI key
DKDILZBBFKZMRO-HNNXBMFYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
L-Histidine β-naphthylamide is an analogue of L-histidine used to study structural roles of L-histidine in functions such as the activation of HutP protein transcription. L-Histidine β-naphthylamide may also be used to study the specificity and kinetics of various proteases and peptidases.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R A Field et al.
The Biochemical journal, 274 ( Pt 3), 885-889 (1991-03-15)
This present study reports the ability of a range of derivatives of L-histidine, histamine and imidazole to act as inhibitors of sweet-almond beta-glucosidase, yeast alpha-glucosidase and Escherichia coli beta-galactosidase. The addition of a hydrophobic group to the basic imidazole nucleus
X Iturrioz et al.
Biochemistry, 39(11), 3061-3068 (2000-03-15)
Aminopeptidase A (EC 3.4.11.7, APA) is a 130 kDa membrane-bound protease that contains the HEXXH consensus sequence found in the zinc metalloprotease family, the zincins. In addition to the catalytic zinc atom, APA contains a Ca2+ ion that increases its
Allosteric activation of HutP protein, that regulates transcription of hut operon in Bacillus subtilis, mediated by various analogs of histidine.
Kumarevel T, Mizuno H, Kumar PK.
Nucleic Acids Research, 3, 199-200 (2003)
Endang Setyorini et al.
Journal of basic microbiology, 46(4), 294-304 (2006-07-19)
A halotolerant strain FP-133, able to grow at concentrations of 0-12.5% (w/v) NaCl, was isolated from a fish paste and identified as Bacillus subtilis . B. subtilis strain FP-133 produced an intracellular protease which showed catalytic activity under saline conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service