All Photos(1)
About This Item
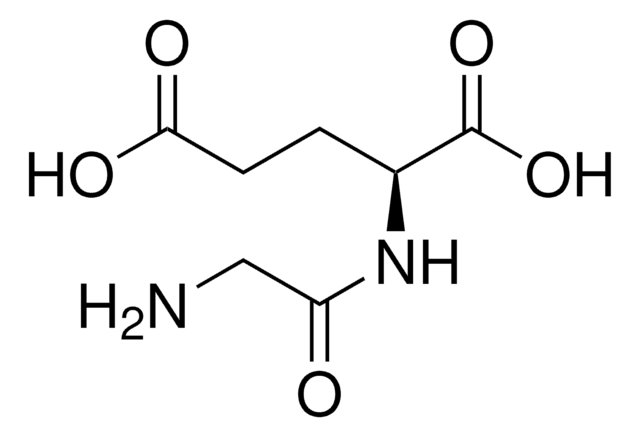
Empirical Formula (Hill Notation):
C11H21N3O5
CAS Number:
Molecular Weight:
275.30
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
γ-Glu-ε-Lys,
Assay
≥98.0% (TLC)
Quality Level
form
powder
color
white
storage temp.
−20°C
SMILES string
NC(CCCCNC(=O)CCC(N)C(O)=O)C(O)=O
InChI
1S/C11H21N3O5/c12-7(10(16)17)3-1-2-6-14-9(15)5-4-8(13)11(18)19/h7-8H,1-6,12-13H2,(H,14,15)(H,16,17)(H,18,19)
InChI key
JPKNLFVGUZRHOB-UHFFFAOYSA-N
Application
GammaGlu-epsilonLys (γ-Glu-ε-Lys) is used to study the functions and processing of endo-epsilon(-gamma-Glu)-Lys isopeptide bonds in biological processes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Julian L Wong et al.
Development (Cambridge, England), 136(11), 1835-1847 (2009-05-01)
Fertilization is accompanied by the construction of an extracellular matrix that protects the new zygote. In sea urchins, this structure is built from glycoproteins residing at the egg surface and in secretory vesicles at the egg cortex. Four enzymatic activities
L Zavalova et al.
Molecular & general genetics : MGG, 253(1-2), 20-25 (1996-11-27)
We previously detected in salivary gland secretions of the medicinal leech (Hirudo medicinalis) a novel enzymatic activity, endo-epsilon(gamma-Glu)-Lys isopeptidase, which cleaves isopeptide bonds formed by transglutaminase (Factor XIIIa) between glutamine gamma-carboxamide and the epsilon-amino group of lysine. Such isopeptide bonds
Damien M O Halloran et al.
Tissue engineering, 12(6), 1467-1474 (2006-07-19)
This study investigated the effect on the mechanical and physicochemical properties of type II collagen scaffolds after cross-linking with microbial transglutaminase (mTGase). It is intended to develop a collagen-based scaffold to be used for the treatment of degenerated intervertebral discs.
I P Baskova et al.
Biochemistry. Biokhimiia, 66(12), 1368-1373 (2002-01-29)
Destabilase, endo-epsilon-(gamma-Glu)-Lys-isopeptidase, was prepared from the salivary gland secretion of the medicinal leech (Hirudo medicinalis). The secretion prepared by the known method of Rigbi et al. (1987) (secretion-K) lacks the destabilase-characteristic highly specific isopeptidase activity (the D-dimer-monomerizing activity) because of
Thomas M Jeitner et al.
Amino acids, 44(1), 129-142 (2012-03-13)
Transglutaminases catalyze the formation of γ-glutamylamines utilizing glutamyl residues and amine-bearing compounds such as lysyl residues and polyamines. These γ-glutamylamines can be released from proteins by proteases in an intact form. The free γ-glutamylamines can be catabolized to 5-oxo-L-proline and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service