A9891
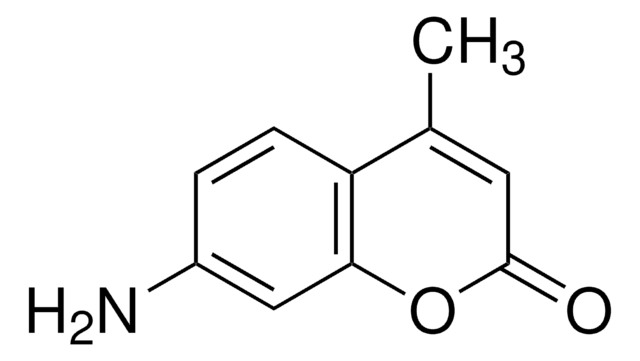
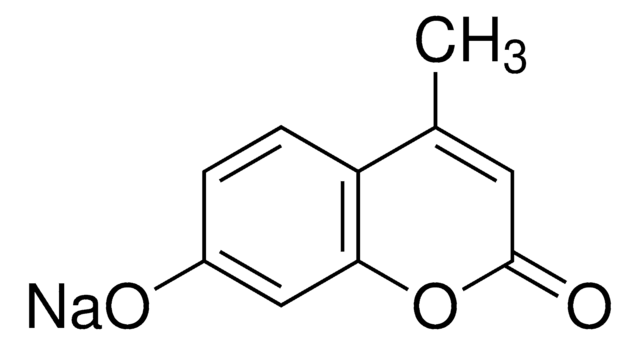
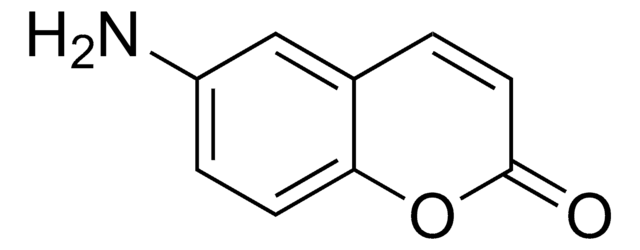
7-Amino-4-methylcoumarin
chromophore for enzyme substrates, fluorogenic, ≥98% (HPLC), powder
Synonym(s):
Coumarin 120
About This Item
Recommended Products
Product Name
7-Amino-4-methylcoumarin, Chromophore for substrates
description
chromophore for enzyme substrates
Assay
≥98% (HPLC)
form
powder
mp
223-226 °C (lit.)
solubility
acetone: 10 mg/mL, clear, colorless to yellow
fluorescence
λex 365 nm; λem 440 nm in ethanol(lit.)
storage temp.
2-8°C
SMILES string
CC1=CC(=O)Oc2cc(N)ccc12
InChI
1S/C10H9NO2/c1-6-4-10(12)13-9-5-7(11)2-3-8(6)9/h2-5H,11H2,1H3
InChI key
GLNDAGDHSLMOKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- to determine cathepsin B like- activity
- as a substrate for leucine aminopeptidase (LAP)
- in chymotryptic assay
- as a standard to detect the activation of caspase-3 during
- sanguinarine-induced damage
- protection by human DEAD-box DDX3
Biochem/physiol Actions
Preparation Note
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Glycosylation is known to have profound influence on various physiochemical, cellular and biological functions of proteins. Alterations in this modification are known to affect the immune system and have been associated with various pathological states such as cancer, rheumatoid arthritis, and inflammatory diseases.
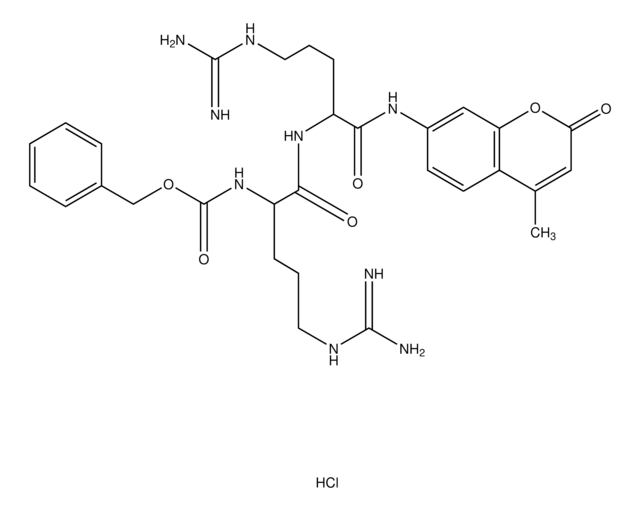
This procedure applies to all products that have a specification for Cathepsin B activity determined by the liberation of 7-amino-4-methylcoumarin from Z-Arg-Arg 7-amido-4-methylcoumarin.
Mass Spectrometry of Glycans, method comparison and products
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service