67138
β-(1→3)-D-Glucanase from Helix pomatia
≥0.2 U/mg
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
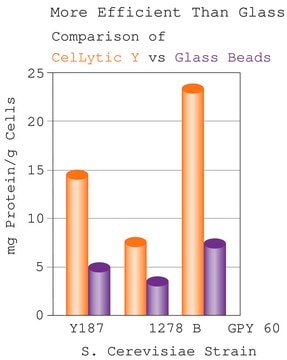
biological source
Helix pomatia
Quality Level
form
powder
specific activity
≥0.2 U/mg
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
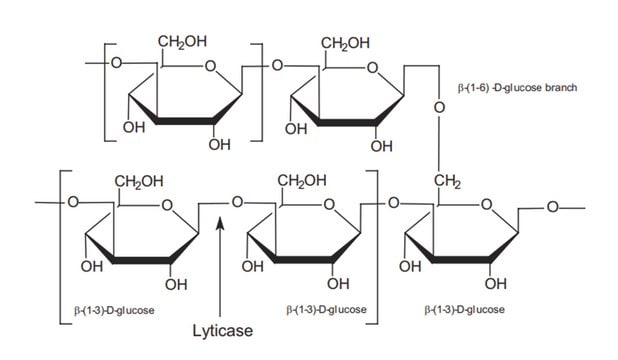
β-(1→3)-D-Glucanase from is used to digest β -1,3-glucan, which is a major component of cell walls. β-(1→3)-D-Glucanase from Helix pomatia has been used fto digest the cell walls of C. albicans .
Biochem/physiol Actions
Deletion of the C.albicans histidine kinase gene (CHK1) improves recognition by phagocytes through an increased exposure of cell wall b-1,3-glucans, which are readily digested by β-(1→3)-D-Glucanases .
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Unit Definition
One unit corresponds to the amount of enzyme which liberates 1 μmol of glucose from laminarin (Cat. No. 61340) per minute at pH 5.0 and 37 °C
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yoichi Tanabe et al.
Biochimica et biophysica acta, 1814(12), 1713-1719 (2011-10-08)
An endo-1,3-β-glucanase was purified from Tunicase®, a crude enzyme preparation from Cellulosimicrobium cellulans DK-1, and determined to be a 383-residue protein (Ala1-Leu383), comprising a catalytic domain of the glycoside hydrolase family 16 and a C-terminal carbohydrate-binding module family 13. The
Enrico Cabib et al.
Eukaryotic cell, 11(4), 388-400 (2012-03-01)
Previous results suggested that the chitin ring present at the yeast mother-bud neck, which is linked specifically to the nonreducing ends of β(1-3)glucan, may help to suppress cell wall growth at the neck by competing with β(1-6)glucan and thereby with
Marián Mazáň et al.
The Biochemical journal, 438(2), 275-282 (2011-06-10)
BGTs [β-(1,3)-glucanosyltransglycosylases; EC 2.4.1.-] of the GH72 (family 72 of glycosylhydrolases) are GPI (glycosylphosphatidylinositol)-anchored proteins that play an important role in the biogenesis of fungal cell walls. They randomly cleave glycosidic linkages in β-(1,3)-glucan chains and ligate the polysaccharide portions
Nina Klippel et al.
Microbiology (Reading, England), 156(Pt 11), 3432-3444 (2010-08-07)
The pathogenic fungus Candida albicans is able to cover its most potent proinflammatory cell wall molecules, the β-glucans, underneath a dense mannan layer, so that the pathogen becomes partly invisible for immune cells such as phagocytes. As the C. albicans
Poonam Gautam et al.
Mycopathologia, 172(5), 331-346 (2011-07-15)
Artemisinin, an antimalarial drug, and its derivatives are reported to have antifungal activity against some fungi. We report its antifungal activity against Aspergillus fumigatus (A. fumigatus), a pathogenic filamentous fungus responsible for allergic and invasive aspergillosis in humans, and its
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service