Recommended Products
description
formerly Dowex Marathon™ A2 chloride
Quality Level
quality
monodispersed
moisture
45-54%
technique(s)
LPLC: suitable
matrix
styrene-divinylbenzene (gel)
matrix active group
dimethylethanolamine
particle size
27-34 mesh
440-560 μm
capacity
1.2 meq/mL by wetted bed volume
separation technique
anion exchange
Related Categories
General description
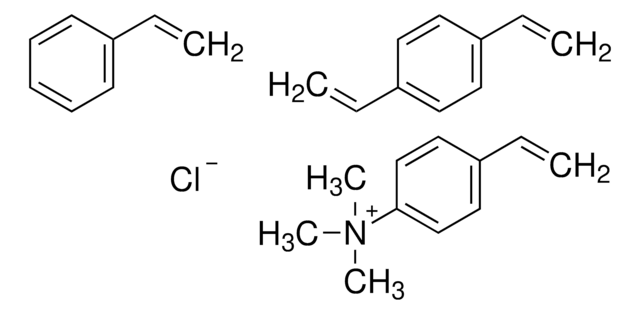
AmberLite™HPR4100 Cl has dimethylethanolamine as functional group.
Application
AmberLite™ HPR4100 Cl was used in structural characterization of Fe in dilute natural waters.
Strongly basic (type II) anion exchange resin well suited for water with high concentration of mineral acids (chlorides, sulfates) and low concentration of silica and CO2 (<25%). This resin has excellent efficiency for general demineralization, and meets requirements of FDA Food Additive Regulation 21 CFR 173.25.
Features and Benefits
Strongly basic anion
Other Notes
This ion-exchange resin has not been specially processed nor cleaned. We suggest that it be treated to a suitable preliminary elution and wash.
Legal Information
Amberlite is a trademark of DuPont de Nemours, Inc.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anneli Sundman et al.
Environmental science & technology, 47(15), 8557-8564 (2013-07-03)
The properties of iron (Fe) complexes and compounds in the environment influence several central processes, e.g., iron uptake, adsorption/desorption of contaminants and nutrients, and redox transformations, as well as the fate of of natural organic matter (NOM). It is thus
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service