All Photos(2)
About This Item
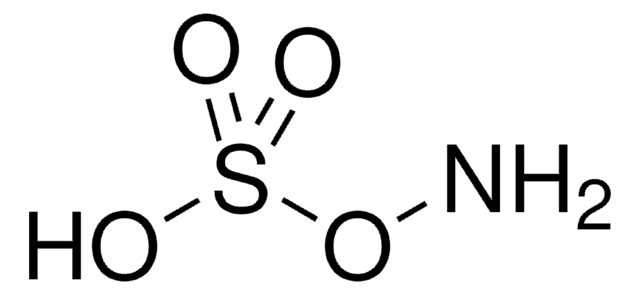
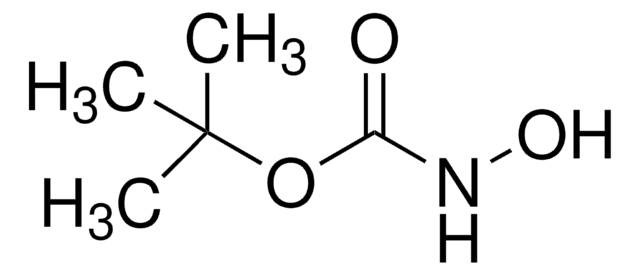
Linear Formula:
H2NOSO3H
CAS Number:
Molecular Weight:
113.09
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
97%
mp
210 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
NOS(O)(=O)=O
InChI
1S/H3NO4S/c1-5-6(2,3)4/h1H2,(H,2,3,4)
InChI key
DQPBABKTKYNPMH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hydroxylamine-O-sulfonic acid is a versatile synthetic reagent. It is widely employed as a nucleophile and as an electrophile in various organic syntheses. It can be synthesized by reacting hydroxylamine sulfate with 30% fuming H2SO4.
Application
Hydroxylamine-O-sulfonic acid (HOSA) may be employed in the preparation of the following:
- monosubstituted and 1,1-disubstituted hydrazines
- symmetrically substituted pyrroles
- aniline
- C-substituted amino derivatives
- lactams
Hydroxylamine-O-sulfonic acid may be used in the following processes:
As a nitrogen transfer reagent for the conversion of aldehydes to nitriles in aqueous medium.
Synthesis of N-aminopiperidine (NAPP) by reacting with piperidine.
As a nitrogen transfer reagent for the conversion of aldehydes to nitriles in aqueous medium.
Synthesis of N-aminopiperidine (NAPP) by reacting with piperidine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Direct synthesis of nitriles from aldehydes with hydroxylamine-O-sulfonic acid in acidic water.
Quinn DJ, et al.
Tetrahedron Letters, 57(34), 3844-3847 (2016)
A New Strategy for the Preparation of N-Aminopiperidine Using Hydroxylamine-O-Sulfonic Acid: Synthesis, Kinetic Modelling, Phase Equilibria, Extraction and Processes."
Labarthe E, et al.
Advances in Chemical Engineering and Science, 3(2) (2013)
Hydroxylamine-O-sulfonic Acid.
Erdik E and Saczewski J.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2013)
Synthetic Methods and Reactions; 671. One-Step Conversion of Alicyclic Ketones into Lactams with Hydroxylamine-O-sulfonic Acid/Formic Acid.
Olah GA and Fung AP.
Synthesis, 07, 537-538 (1979)
K Sakano et al.
Mutation research, 479(1-2), 101-111 (2001-07-27)
2-Nitropropane (2-NP), a widely used industrial solvent, is carcinogenic to rats. To clarify the mechanism of carcinogenesis by 2-NP, we investigated DNA damage by 2-NP metabolites, N-isopropylhydroxylamine (IPHA) and hydroxylamine-O-sulfonic acid (HAS), using 32P-5'-end-labelled DNA fragments obtained from genes that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service