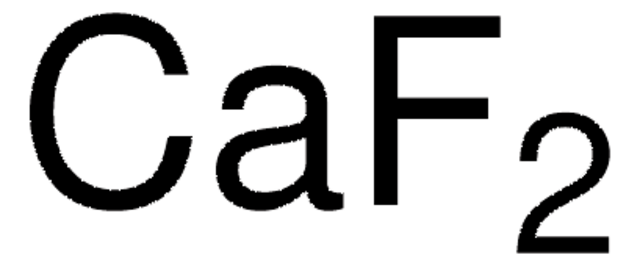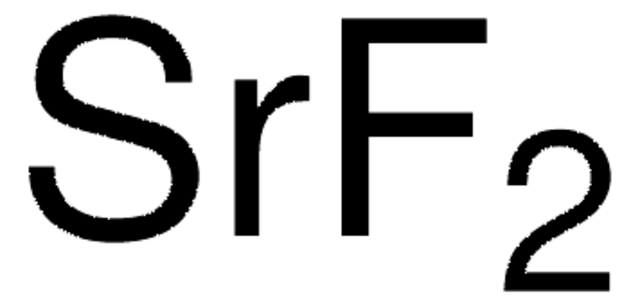05-0670
Calcium fluoride
SAJ first grade, ≥97.0%
Synonym(s):
Fluorite, Fluorspar
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
CaF2
CAS Number:
Molecular Weight:
78.07
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
Recommended Products
grade
SAJ first grade
Assay
≥97.0%
form
powder
availability
available only in Japan
bp
2500 °C (lit.)
density
3.18 g/mL at 25 °C (lit.)
SMILES string
F[Ca]F
InChI
1S/Ca.2FH/h;2*1H/q+2;;/p-2
InChI key
WUKWITHWXAAZEY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jason S Lupoi et al.
Biotechnology for biofuels, 8, 98-98 (2015-07-23)
Slow-degrading, fossil fuel-derived plastics can have deleterious effects on the environment, especially marine ecosystems. The production of bio-based, biodegradable plastics from or in plants can assist in supplanting those manufactured using fossil fuels. Polyhydroxybutyrate (PHB) is one such biodegradable polyester
J M ten Cate
European journal of oral sciences, 105(5 Pt 2), 461-465 (1997-12-12)
Low concentrations of fluoride have a beneficial effect on enamel and dentin de- and remineralization. After fluoride treatments, such as topical applications, rinses or dentifrices, salivary fluoride concentrations decrease exponentially in a biphasic manner to very low concentrations within a
G Rølla
Acta odontologica Scandinavica, 46(6), 341-345 (1988-12-01)
The literature concerning the formation and stability of CaF2 in the oral environment is reviewed. In early work the CaF2 formed during topical application with fluoride was assumed to be beneficial. It was suggested that it could protect the enamel
Wade R Hirschman et al.
Journal of endodontics, 38(3), 385-388 (2012-02-22)
The purpose of this in vitro study was to compare the cytotoxicity of white mineral trioxide aggregate cement (AMTA, MTA-Angelus), Brasseler Endosequence Root Repair Putty (ERRM), Dycal, and Ultra-blend Plus (UBP) by using human dermal fibroblasts and a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
Silvane Morés et al.
Talanta, 85(5), 2681-2685 (2011-10-04)
High-resolution continuum source molecular absorption of the calcium mono-fluoride molecule CaF in a graphite furnace has been used to determine fluorine in tea after acid digestion, alkaline solubilization and preparation of a conventional aqueous infusion. The strongest absorption 'line' of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service