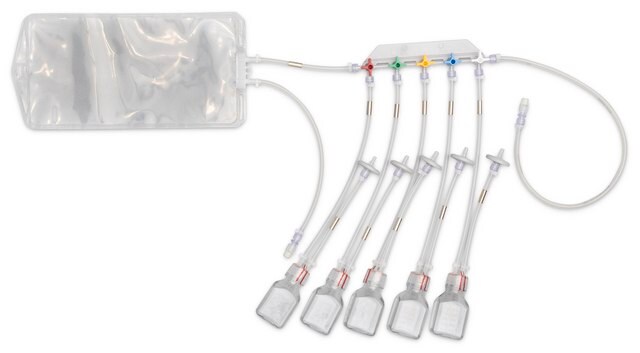
E9SU871
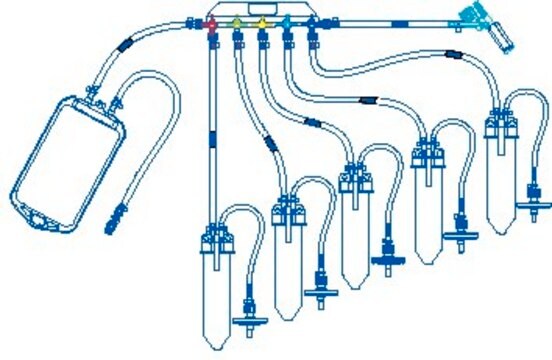
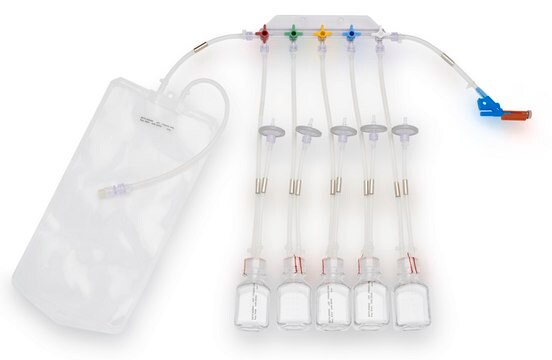
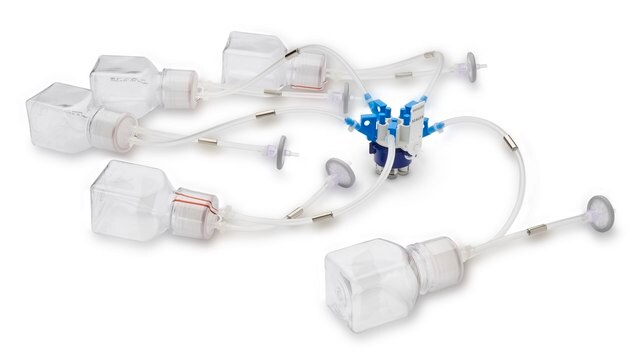
NovaSeptum® GO Preloaded System with Bottle, 9-port
sterile; γ-irradiated
Synonym(s):
NovaSeptum® GO Bottle
About This Item
Recommended Products
material
316 stainless steel cannula
PETG bottle (fluid contact layer)
TPE tubing
polyester body
silicone septum (platinum-cured)
Quality Level
Agency
according to ISO 11137 (sterilization)
meets ISO 146441
146441
meets ISO 88 biological reactivity (all component materials)
sterility
sterile; γ-irradiated
product line
NovaSeptum® GO
feature
autoclavable: no
parameter
-80-50 °C temp. range (-112-122 °F)
0.50 bar max. pressure (7.25 psi)
impurities
<2.15 EU/device bacterial endotoxins (LAL test)
application(s)
cell therapy
gene therapy
life science and biopharma
mAb
ophthalmics
parenterals
pathogen testing
pharma/biopharma processes
sterile sampling
viral therapy
storage temp.
room temp
Related Categories
General description
Application
Bioburden - Archiving
Packaging
E9SU871-80125 (9x125 ml bottles, 2 units per box)
E9SU871-80250 (9x250 ml bottles, 1 units per box)
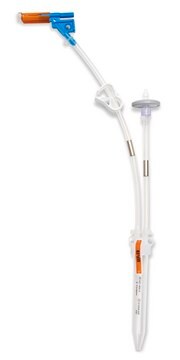
Components
- Septum: Platinum-cured silicone
- Body: Polyester
- Cannula: ASTM® 316 L Stainless steel
- Fluid contact layer (bottle): Polyethylene Terephtalate Glycol
- Tubing: Thermoplastic elastomer (TPE)
- Outlet Tubing: Cap
Preparation Note
Aqueous extraction contains < 2.15 EU per device as determined using the Limulus Amebocyte Lysate (LAL) test.
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service