T84964
Tripropargylamine
98%
Synonym(s):
Tris(2-propynyl)amine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
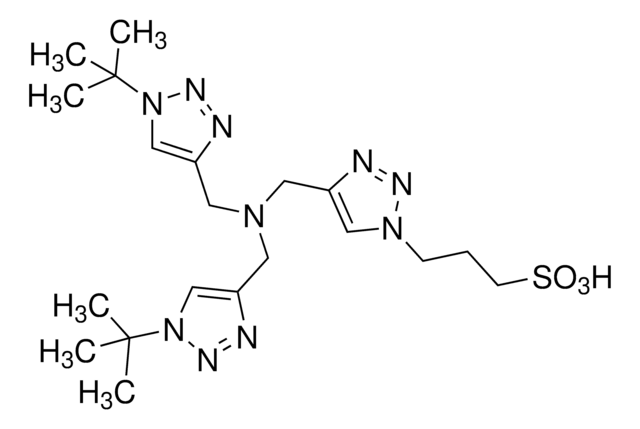
Linear Formula:
(HC≡CCH2)3N
CAS Number:
Molecular Weight:
131.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.484 (lit.)
bp
79-85 °C/11 mmHg (lit.)
density
0.927 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C#CCN(CC#C)CC#C
InChI
1S/C9H9N/c1-4-7-10(8-5-2)9-6-3/h1-3H,7-9H2
InChI key
ZHOBJWVNWMQMLF-UHFFFAOYSA-N
Related Categories
Application
Tripropargylamine is widely used in the synthesis of Cu(I) stabilizing ligands such as tris(3-hydroxypropyltriazolylmethyl)amine (THPTA) and tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) for the copper-catalyzed alkyne-azide cycloaddition (CuAAC) click reaction.
It can also be used in the synthesis of anthracene appended tripodal triazole, tris[(9-anthryl benzyl-1H-1,2,3-triazole-4-yl)methyl]amine as a receptor for fluorescence recognition of Ni2+ in aqueous solution.
It can also be used in the synthesis of anthracene appended tripodal triazole, tris[(9-anthryl benzyl-1H-1,2,3-triazole-4-yl)methyl]amine as a receptor for fluorescence recognition of Ni2+ in aqueous solution.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
134.6 °F - closed cup
Flash Point(C)
57 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Click grafting of alkyne-containing vinyl polymers onto biosynthesized extracellular matrix protein containing azide functionality and adhesion control of human umbilical vein endothelial cells.
Yamada T and Takasu A
Royal Society of Chemistry Advances, 5(52), 41445-41456 (2015)
Analysis and Optimization of Copper?Catalyzed Azide?Alkyne Cycloaddition for Bioconjugation.
Hong V, et al.
Angewandte Chemie (International Edition in English), 48(52), 9879-9883 (2009)
A new click-derived tripodal receptor for fluorescence recognition of Ni2+ in aqueous solution.
Zhu J H, et al.
Inorgorganica Chimica Acta, 451, 111-115 (2016)
Cu (II)-Catalyzed Oxidative Formation of 5, 5′-Bistriazoles.
Brassard C J, et al.
The Journal of Organic Chemistry, 81(24), 12091-12105 (2016)
Qian Zhang et al.
ChemistryOpen, 8(5), 571-579 (2019-05-09)
An azide terminated ethylene oxide-tetrahydrofuran copolymer with urethane segments (ATUPET) as a novel binder pre-polymer, has been prepared through ethylene oxide-tetrahydrofuran random copolymer (PET) end-capping modification via one-pot method. The structure characterization of the modifier has been analyzed by FTIR
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)