F6129
Iron(III) citrate
technical grade
Synonym(s):
Ferric citrate, Iron(III) citrate, Iron(III) citrate hydrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
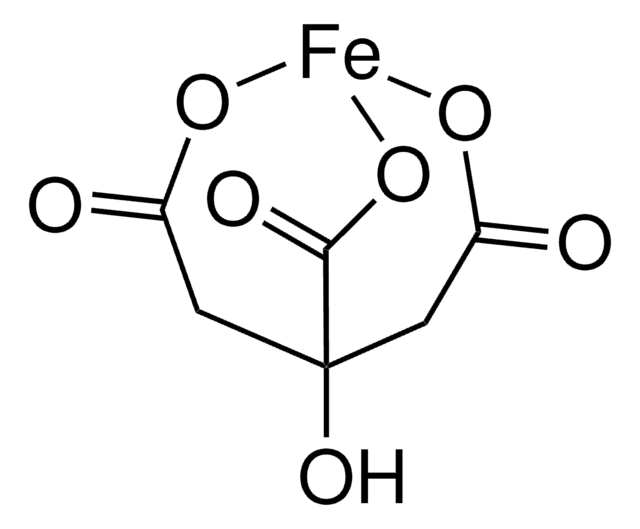
C6H5FeO7
CAS Number:
Molecular Weight:
244.94
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
technical grade
form
powder
composition
Fe, 16.5-18.5%
technique(s)
cell culture | mammalian: suitable
application(s)
battery manufacturing
SMILES string
OC12CC(=O)O[Fe](OC(=O)C1)OC2=O
InChI
1S/C6H8O7.Fe/c7-3(8)1-6(13,5(11)12)2-4(9)10;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);/q;+3/p-3
InChI key
NPFOYSMITVOQOS-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Iron(III) citrate technical grade is a brown to dark orangepowder. It is soluble in water, but insoluble in most organics includingalcohols. The powder is sensitive to light; like many iron carboxylates, bluelight photo-reduces iron(III) citrate, forming the Fe2+ ion andconcomitantly oxidizing the carboxyl group to yield carbon dioxide. Iron(III)citrate thermally decomposes to α-Fe2O3 at 460 °C.
Application
Iron(III) citrate is a synthetic precursor for iron-containing compounds. It is commonly used to prepare Fe3O4 nanoparticles or Fe3O4-nanocomposites by hydrothermal methods and Fe2O3 materials by thermal decomposition and sol-gel processing. Iron(III) citrate is well-suited to sol-gel processing because of its high solubility in water and low solubility inorganic phases. Consequently, iron(III) citrate is an important precursor in the synthesis of iron-doped and iron-containing metal oxides studied for lithium-ion battery cathodes.
It can also be used in the degradation of tetracycline for the treatment of polluted water.
It can also be used in the degradation of tetracycline for the treatment of polluted water.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Senthil Kumar et al.
ACS omega, 3(3), 3036-3044 (2018-07-20)
Pristine trivanadate (LiV3O8) and doped lithium trivanadate (LiV3-x M x O8, M = Zn/Co/Fe/Sn/Ti/Zr/Nb/Mo, x = 0.01/0.05/0.1 M) compounds were prepared by a simple reflux method in the presence of the polymer, Pluronic P123, as the chelating agent. For comparison
Jianguo Guan et al.
Chemical communications (Cambridge, England), 46(35), 6605-6607 (2010-08-18)
We present a simple and effective heterogeneous contraction method to fabricate hollow spheres with controllable interior structures (ranging from solid, simple hollow to core-in-hollow-wall, double-wall hollow and core-in-double-hollow-wall spheres) by a non-equilibrium heat-treatment process of gel precursors with a high
Petra Vukosav et al.
Analytica chimica acta, 745, 85-91 (2012-09-04)
A detailed study of iron (III)-citrate speciation in aqueous solution (θ=25°C, I(c)=0.7 mol L(-1)) was carried out by voltammetric and UV-vis spectrophotometric measurements and the obtained data were used for reconciled characterization of iron (III)-citrate complexes. Four different redox processes
Marvin Sinsakul et al.
Nephron. Clinical practice, 121(1-2), c25-c29 (2012-10-19)
A phase II open-label study was conducted in hemodialysis patients evaluating the short-term safety, tolerability, and iron absorption with ferric citrate when used as a phosphate binder. Enrollment occurred in two periods. Period 1 recruited patients taking 6-15 pills/day of
Hiroaki Ito et al.
The journal of physical chemistry. A, 115(21), 5371-5379 (2011-05-13)
The kinetics of ligand exchange between ferric citrate and desferrioxamine B (DFB) was investigated at pH 8.0 and high citrate/Fe molar ratios (500-5000) with particular attention given to understanding the precise mechanism of ligand exchange. Ferric citrate complexes present in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service