All Photos(1)
About This Item
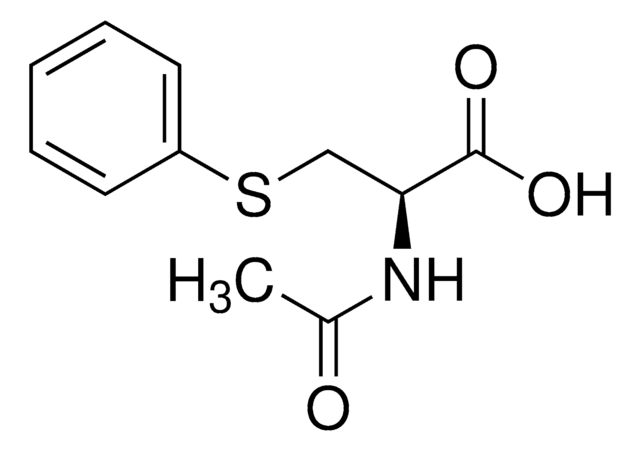
Linear Formula:
C6H5CH2SCH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
211.28
Beilstein:
1879358
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
optical activity
[α]20/D +23°, c = 2 in 1 M NaOH
reaction suitability
reaction type: solution phase peptide synthesis
color
white to off-white
mp
214 °C (dec.) (lit.)
application(s)
detection
peptide synthesis
SMILES string
N[C@@H](CSCc1ccccc1)C(O)=O
InChI
1S/C10H13NO2S/c11-9(10(12)13)7-14-6-8-4-2-1-3-5-8/h1-5,9H,6-7,11H2,(H,12,13)/t9-/m0/s1
InChI key
GHBAYRBVXCRIHT-VIFPVBQESA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Michelle Rudden et al.
Scientific reports, 10(1), 12500-12500 (2020-07-29)
Body odour is a characteristic trait of Homo sapiens, however its role in human behaviour and evolution is poorly understood. Remarkably, body odour is linked to the presence of a few species of commensal microbes. Herein we discover a bacterial
M Rooseboom et al.
Chemical research in toxicology, 14(1), 127-134 (2001-02-15)
Previously, it was shown that beta-elimination of selenocysteine Se-conjugates by rat renal cytosol leading to pyruvate formation was not solely catalyzed by pyridoxal phosphate-dependent enzymes. It was hypothesized that selenoxidation of the selenocysteine Se-conjugates, followed by syn-elimination, may be an
M Inoue et al.
Journal of biochemistry, 95(1), 247-254 (1984-01-01)
Biosynthesis of N-acetylcysteine S-conjugates of xenobiotics, mercapturic acids, occurs via inter-organ metabolism of the corresponding glutathione derivatives (Inoue, M., Okajima, K., & Morino, Y. (1982) Hepatology 2, 311-316). To elucidate the mechanism of mercapturate biosynthesis and its urinary elimination, hepato-renal
A potent pyridoxal model capable of promoting transamination and beta-elimination of amino acids.
H Kondo et al.
Progress in clinical and biological research, 144A, 101-108 (1984-01-01)
K Okajima et al.
European journal of biochemistry, 142(2), 281-286 (1984-07-16)
Acetylation of cysteine S-conjugates of xenobiotics by microsomal N-acetyltransferase is the final step of detoxicative metabolism leading to mercapturic acid biosynthesis. To elucidate the subcellular site of N-acetylation and the effective mechanism by which the final metabolites are eliminated from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service