682071
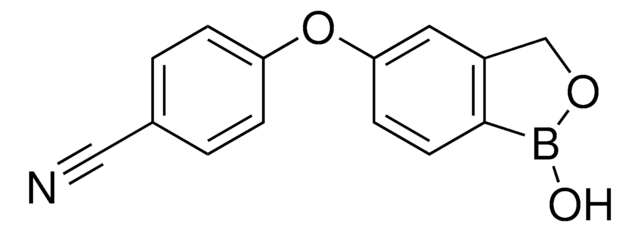
2-(Hydroxymethyl)phenylboronic acid cyclic monoester
97%
Synonym(s):
1,3-Dihydro-1-hydroxy-2,1-benzoxaborole, 2-(Hydroxymethyl)phenylboronic acid hemiester
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H7BO2
CAS Number:
Molecular Weight:
133.94
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
95-100 °C
SMILES string
OB1OCc2ccccc12
InChI
1S/C7H7BO2/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4,9H,5H2
InChI key
XOQABDOICLHPIS-UHFFFAOYSA-N
Related Categories
Application
Oxaboroles and benzoxaboroles are useful substrates to prepare allylic and benzylic alcohols via Suzuki coupling.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Eric C Hansen et al.
Journal of the American Chemical Society, 128(25), 8142-8143 (2006-06-22)
A novel mode of regiochemical control over the allylic [1,3]-transposition of silyloxy groups catalyzed by Re2O7 has been developed. This strategy relies on a cis-oriented vinyl boronate, generated from the Alder-ene reaction of homoallylic silyl ethers and alkynyl boronates, to
Synthesis, 1148-1148 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service