641472
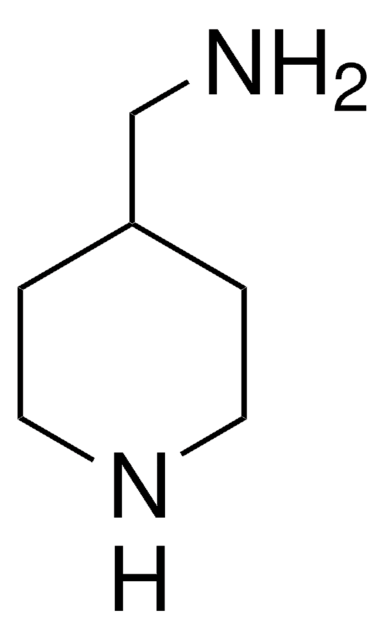
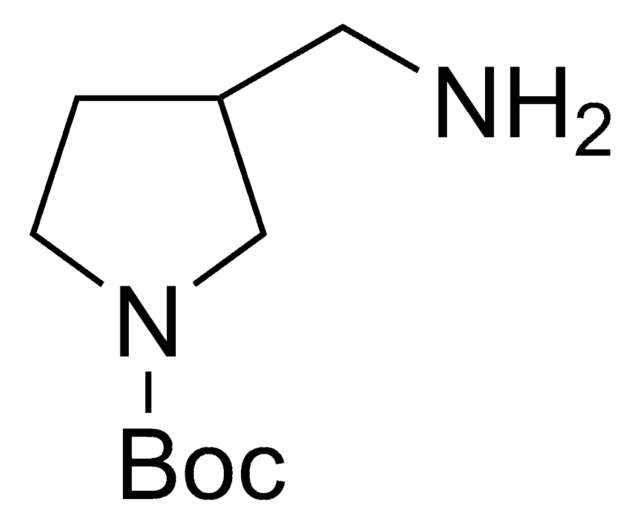
1-Boc-4-(aminomethyl)piperidine
97%
Synonym(s):
1,1-Dimethylethyl 4-(aminomethyl)-1-piperidinecarboxylate, N-(tert-Butoxycarbonyl)-4-aminomethylpiperidine
About This Item
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.473 (lit.)
bp
237-238 °C (lit.)
density
1.013 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CC(C)(C)OC(=O)N1CCC(CN)CC1
InChI
1S/C11H22N2O2/c1-11(2,3)15-10(14)13-6-4-9(8-12)5-7-13/h9H,4-8,12H2,1-3H3
InChI key
KLKBCNDBOVRQIJ-UHFFFAOYSA-N
Related Categories
Application
- Kinesin spindle protein inhibitors with potential anticancer activity
- Orphan G-protein coupled receptor GPR119 agonist with antidiabetic potential
- Pim-1 inhibitors
- Aspartic acid protease inhibitors
Reactant involved in enantioselective synthesis of N-alkyl terminal aziridines
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 641472-25G | 4061833046128 |
| 641472-5G | 4061833046135 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service