All Photos(2)
About This Item
Empirical Formula (Hill Notation):
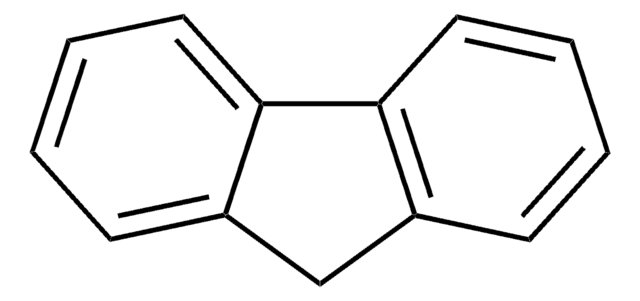
C21H26
CAS Number:
Molecular Weight:
278.43
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
121-124 °C (lit.)
SMILES string
CC(C)(C)c1ccc-2c(Cc3cc(ccc-23)C(C)(C)C)c1
InChI
1S/C21H26/c1-20(2,3)16-7-9-18-14(12-16)11-15-13-17(21(4,5)6)8-10-19(15)18/h7-10,12-13H,11H2,1-6H3
InChI key
DFZYPLLGAQIQTD-UHFFFAOYSA-N
Related Categories
General description
2,7-Di-tert-butylfluorene can be synthesized by reacting fluorene, CS2 and FeCl3 and 2-chloro-2-methylpropane. It can also be obtained by reacting fluorene with tert-butyl chloride in the presence of FeCl3.
Application
2,7-Di-tert-butylfluorene may be used in the preparation of:
- 2,7-di-tert-butyl-9-fluorenylmethanol
- 2,7-di-tert-butyl-9-[ [(p-chlorophenyl)amino]methylene]-fluorene
- dihydrocyclohepta[def]fluorene
- new group 4 metal complexes containing aminofluorenyl ligands
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Miller SA and Bercaw JE.
Organometallics, 19, 5608-5608 (2000)
Synthesis and Properties of Kinetically Stabilized Cyclohepta [def] fluorene Derivatives.
Grieser, UD and Hafner K.
Chemische Berichte, 127(11), 2307-2314 (1994)
Investigation of the reaction between amino acids or amino acid esters and 9-formylfluorene and its equivalents. Possible utility of the derived enamines as amino group protectants.
Carpino LA, et al.
The Journal of Organic Chemistry, 54(18), 4302-4313 (1989)
Fmoc: a more soluble analogue of the 9-fluorenylmethoxycarbonyl protecting group.
K D Stigers et al.
The Journal of organic chemistry, 65(12), 3858-3860 (2000-06-24)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service