All Photos(1)
About This Item
Empirical Formula (Hill Notation):
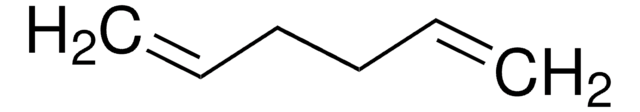
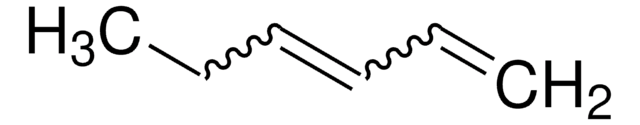
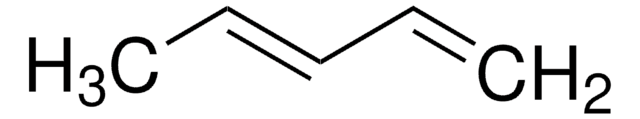
C6H10
CAS Number:
Molecular Weight:
82.14
Beilstein:
1839519
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0%
refractive index
n20/D 1.415
bp
65-66 °C (lit.)
density
0.707 g/mL at 20 °C (lit.)
SMILES string
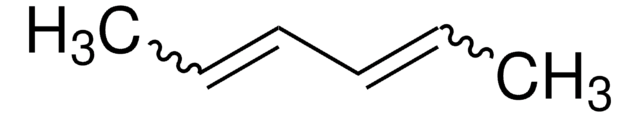
C\C=C/CC=C
InChI
1S/C6H10/c1-3-5-6-4-2/h3-4,6H,1,5H2,2H3/b6-4-
InChI key
PRBHEGAFLDMLAL-XQRVVYSFSA-N
Related Categories
General description
cis-1,4-Hexadiene can be synthesized from the cobalt-catalyzed reaction between butadiene and ethylene. The reaction of this hydrocarbon with Fe(CO)5 affords the trans-1,3-hexadiene-iron tricarbonyl complex (I), plus a small amount of the trans,trans-2,4-hexadiene complex(II). Mechanism of the conversion of cis-1,4-hexadiene to trans-2-methyl-1,3-pentadiene, via rearrangement in the presence of a nickel catalyst has been investigated.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-50.8 °F - closed cup
Flash Point(C)
-46 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reaction of cis-1, 4-Hexadiene with Iron Pentacarbonyl.
Kuji J and Iwamoto M.
Bulletin of the Chemical Society of Japan, 41(6), 1483-1484 (1968)
Nickel-promoted methylvinylcyclopropane rearrangements. Mechanistic relevance to the cis-1, 4-hexadiene to 2-methyl-1, 3-pentadiene isomerization.
Pinke PA, et al.
Journal of the American Chemical Society, 96(13), 4229-4234 (1974)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service