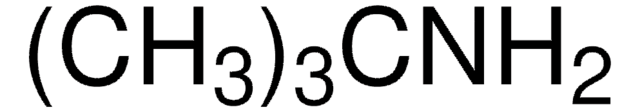All Photos(1)
About This Item
Linear Formula:
(CH3)3CN(CH3)CH(CH3)2
CAS Number:
Molecular Weight:
129.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.419 (lit.)
bp
127 °C (lit.)
density
0.767 g/mL at 25 °C (lit.)
SMILES string
CC(C)N(C)C(C)(C)C
InChI
1S/C8H19N/c1-7(2)9(6)8(3,4)5/h7H,1-6H3
InChI key
WYLLBTPEHIVUKV-UHFFFAOYSA-N
General description
N-Isopropyl-N-methyl-tert-butylamine is a tertiary amine. BH3.THF stabilized by N-isopropyl N-methyl tert-butylamine is useful for the carbonyl reductions.
Application
N-Isopropyl-N-methyl-tert-butylamine may be used to stabilize the borane-THF solutions. It may also be used for the synthesis of rotaxane, via Sonogashira coupling.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
48.2 °F - closed cup
Flash Point(C)
9 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dominic Thibeault et al.
Molecules (Basel, Switzerland), 15(5), 3709-3730 (2010-07-27)
The number of synthetic methods enabling the preparation of ammonium-based rotaxanes has increased very rapidly in the past ten years. The challenge in the synthesis of rotaxanes results from the rather weak interactions between the ammonium-containing rod and the crown
Xiaogen Huang et al.
Organic letters, 9(9), 1793-1795 (2007-04-03)
[reaction: see text] The enantioselective borane reduction of O-benzyloxime ethers to primary amines was studied under catalytic conditions using the spiroborate esters 5-10 derived from nonracemic 1,2-amino alcohols and ethylene glycol. Effective catalytic conditions were achieved using only 10% of
Process Development of a Potent Glucosylceramide Synthase Inhibitor.
Cooper CGF, et al.
Organic Process Research & Development, 16(5), 1090-1097 (2011)
Borane-THF: New Solutions with Improved Thermal Properties and Stability.
Potyen M, et al.
Organic Process Research & Development, 11(2), 210-214 (2007)
Jeffrey A Crank et al.
Journal of the American Society for Mass Spectrometry, 20(10), 1790-1800 (2009-08-12)
Second generation ionic liquid matrices are developed, examined, and tested. They have shown a wide mass detection range (<1000 Da to >270,000 Da) for proteins and peptides with greater S/N ratios than solid matrices. These ionic liquid matrices also exhibit
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service