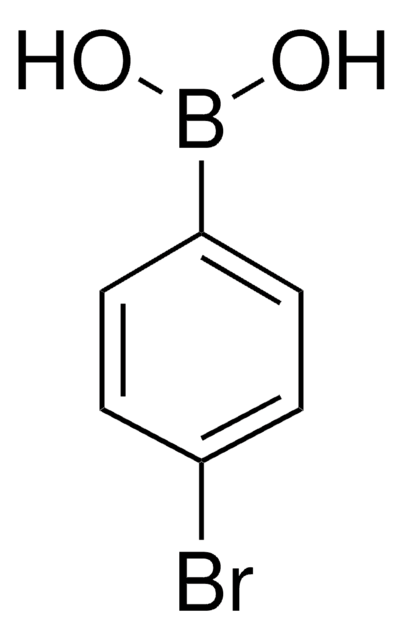
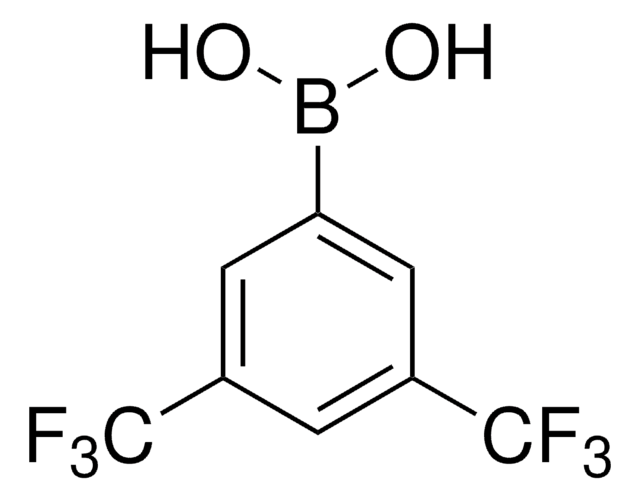
439320
4-(Trifluoromethyl)phenylboronic acid
≥95.0%
Synonym(s):
α,α,α-Trifluoro-p-tolylboronic acid, 4-(Trifluoromethyl)benzeneboronic acid, [p-(Trifluoromethyl)phenyl]boronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CF3C6H4B(OH)2
CAS Number:
Molecular Weight:
189.93
Beilstein:
3544189
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
245-250 °C (lit.)
functional group
fluoro
SMILES string
OB(O)c1ccc(cc1)C(F)(F)F
InChI
1S/C7H6BF3O2/c9-7(10,11)5-1-3-6(4-2-5)8(12)13/h1-4,12-13H
InChI key
ALMFIOZYDASRRC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-(Trifluoromethyl)phenylboronic acid can be used as a reactant in:
It can also be used as a reactant to prepare:
- Site-selective Suzuki-Miyaura cross-coupling reactions.
- Palladium-catalyzed direct arylation reactions.
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence.
- Ruthenium catalyzed direct arylation.
- Ligand-free copper-catalyzed coupling reactions.
- Amination and conjugate addition reactions.
- Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions.
- Rhodium-catalyzed asymmetric 1,4-addition reactions.
- Copper-catalyzed nitration reactions.
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.
- Palladium catalyzed allylation reaction with allyl alcohols.
- N-Arylation of imidazoles and amines in the presence of copper-exchanged fluorapatite as a catalyst.
It can also be used as a reactant to prepare:
- Thiazole derivatives for printable electronics.
- Terphenyl benzimidazoles as tubulin polymerization inhibitors.
- Aryl ketones by cross-coupling reaction with acid chlorides.
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient synthesis of arylated coumarins by site-selective Suzuki-Miyaura cross-coupling reactions of the bis(triflate) of 4-methyl-5,7-dihydroxy-coumarin
Eleya, N.; et al.
Synlett, 23, 223-226 (2012)
S Van Mierloo et al.
Magnetic resonance in chemistry : MRC, 50(5), 379-387 (2012-04-18)
Four 2,5-bis(5-aryl-3-hexylthiophen-2-yl)thiazolo[5,4-d]thiazole derivatives have been synthesized and thoroughly characterized. The extended aromatic core of the molecules was designed to enhance the charge transport characteristics, and solubilizing hexyl side chains were introduced on the thiophene subunits to enable possible integration of
Viktor O Iaroshenko et al.
Organic & biomolecular chemistry, 10(15), 2955-2959 (2012-03-10)
A facile synthetic approach for the synthesis of 1,8-naphthyridine-4(1H)-one derivatives via a catalyst free and Pd-supported tandem amination sequence is developed and described. In a case of aliphatic amines reaction proceeds in a catalyst free mode, however anilines demand Pd-supported
Tetrahedron, 62, 11675-11675 (2006)
M Lakshmi Kantam et al.
The Journal of organic chemistry, 71(25), 9522-9524 (2006-12-02)
N-Arylation of imidazoles and amines with arylboronic acids was accomplished with copper-exchanged fluorapatite (CuFAP) in methanol at room temperature. The products N-arylimidazoles and N-arylamines were isolated in good to excellent yields. A variety of arylboronic acids were converted to the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service