All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H35O2P
CAS Number:
Molecular Weight:
290.42
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Assay
~90% (T)
form
liquid
refractive index
n20/D 1.460
density
0.916 g/mL at 20 °C (lit.)
SMILES string
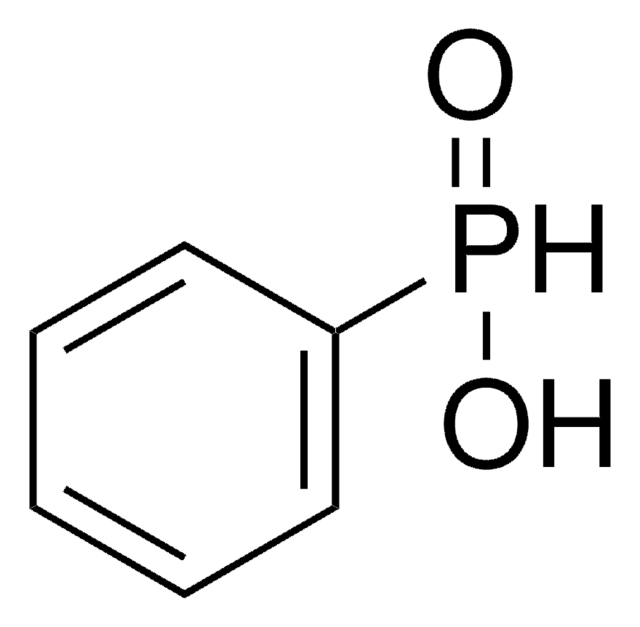
CC(C)CCCCCP(O)(=O)CCCCCC(C)C
InChI
1S/C16H35O2P/c1-15(2)11-7-5-9-13-19(17,18)14-10-6-8-12-16(3)4/h15-16H,5-14H2,1-4H3,(H,17,18)
InChI key
GBNVBFGHGMAMDH-UHFFFAOYSA-N
General description
Diisooctylphosphinic acid is an organophosphorous acid.
Application
Diisooctylphosphinic acid (DiOPA) may be used in the following studies:
- As the surface passivating ligand in the synthesis of magic-sized CdSe and CdTe nanocrystals.
- As extractant in the extraction of Indium(III) from sulfate solutions.
- As ligand to investigate the solubility of four copper ligand systems in supercritical CO2.
- As acid ligand for the Supercritical fluid extraction (SFE) of divalent metals (Zn2+, Cu2+, Pb2+, Cd2+ and Cr3+) from sand and fly ash using CO2.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
226.4 °F - closed cup
Flash Point(C)
108 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Extraction of heavy metals from fly ash and sand with ligands and supercritical carbon dioxide.
Kersch C, et al.
Industrial & Engineering Chemistry Research, 39(12), 4670-4672 (2000)
Synthesis of magic-sized CdSe and CdTe nanocrystals with diisooctylphosphinic acid.
Dukes III AD, et al.
Chemistry of Materials, 22(23), 6402-6408 (2010)
De la Rosa JR, et al.
Materials and Manufacturing Processes, 26(2), 304-310 (2011)
Extraction of indium (III) from sulfate solutions with organophosphorus acids.
Travkin VF, et al.
Russian Journal of Applied Chemistry, 77(10), 1613-1617 (2004)
Taeyjuana Y Lyons et al.
Langmuir : the ACS journal of surfaces and colloids, 33(12), 3018-3027 (2017-03-01)
The increasing commercialization of consumer electronic products that make use of II-VI semiconductor quantum dots (QDs) has raised significant concerns about their impact on natural systems and human health once they are released into the environment at the end of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service