374598
Cetyltrimethylammonium hydrogensulfate
99%
Synonym(s):
CTAHSO4, Hexadecyltrimethylammonium hydrogen sulfate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)15N(HSO4)(CH3)3
CAS Number:
Molecular Weight:
381.61
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
250-260 °C (dec.) (lit.)
SMILES string
OS([O-])(=O)=O.CCCCCCCCCCCCCCCC[N+](C)(C)C
InChI
1S/C19H42N.H2O4S/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(2,3)4;1-5(2,3)4/h5-19H2,1-4H3;(H2,1,2,3,4)/q+1;/p-1
InChI key
UCRJJNVFJGKYQT-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Cetyltrimethylammonium hydrogensulfate is commonly used as a surfactant for organic transformation in the aqueous medium.
Some of the applications include:
Some of the applications include:
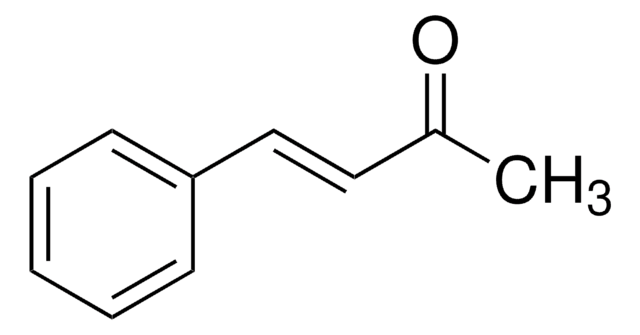
- Rhodium(I)-catalyzed asymmetric hydrogenation of (Z)-methyl-α-acetamidocinnamate.
- Asymmetric palladium-catalyzed alkylation of 1,3-diphenyl-2-propenyl acetate with dimethyl malonate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Catalytic asymmetric alkylation in water in the presence of surfactants
Sinou D, et al.
advanced synthesis and catalysis, 345(3), 357-363 (2003)
Influence of different types of amphiphiles on the rhodium (I) complex-catalyzed asymmetric hydrogenation of (Z)-methyl-α-acetamidocinnamate in aqueous medium
Grassert I, et al.
Tetrahedron, 49(30), 6605-6612 (1993)
Micellar effects upon oxidation of organic sulfides by anionic oxidants
Bacaloglu R, et al.
Journal of Physical Organic Chemistry, 5(4), 171-178 (1992)
Ahmet Gürses et al.
TheScientificWorldJournal, 2012, 270452-270452 (2013-02-01)
The aim of this study was the preparation of polyethylene oxide (PEO)/clay nanocomposites using organoclay produced via micellar adsorption of cethyltrimethyl ammonium bromide (CTAB) and their characterisation by X-ray diffraction (XRD), and Fourier transform infrared (FT-IR) spectra, and the investigation
Lin Wang et al.
Journal of hazardous materials, 244-245, 681-688 (2012-11-28)
Photocatalytic reduction of aqueous Cr(VI) was successfully achieved on nanostructured SnIn(4)S(8). The SnIn(4)S(8) particles with flower-like nanostructure were synthesized via a facile solvothermal method. UV-vis diffuse reflectance spectra (DRS) indicated that the SnIn(4)S(8) particles had strong absorption in visible region
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service