All Photos(1)
About This Item
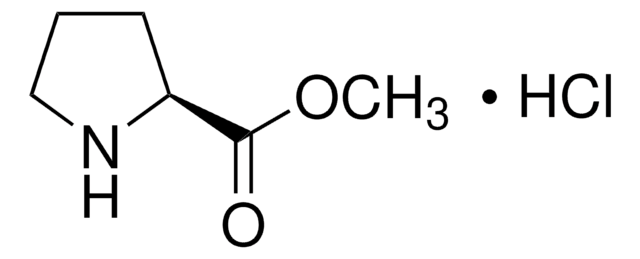
Empirical Formula (Hill Notation):
C12H15NO2 · HCl
CAS Number:
Molecular Weight:
241.71
Beilstein:
3598081
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
optical activity
[α]20/D −48°, c = 1 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
148-151 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl[H].[H][C@]1(CCCN1)C(=O)OCc2ccccc2
InChI
1S/C12H15NO2.ClH/c14-12(11-7-4-8-13-11)15-9-10-5-2-1-3-6-10;/h1-3,5-6,11,13H,4,7-9H2;1H/t11-;/m0./s1
InChI key
NEDMOHHWRPHBAL-MERQFXBCSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Extensive applications in peptide chemistry and used as a chiral auxiliary in the asymmetric Diels-Alder reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Córdova et al.
Bioorganic & medicinal chemistry letters, 9(21), 3119-3122 (1999-11-24)
Mesylates or tosylates of delta-hydroxy-L-norvaline esters spontaneously afford L-proline esters upon exposure to aqueous buffer in near quantitative yield. This novel reaction has led to the development of a simple route to optically active proline esters.
Enantiomeric purity determination of L-proline benzyl ester by chiral column gas chromatography.
M Jemal et al.
Journal of chromatography, 392, 442-446 (1987-04-17)
Aldrichimica Acta, 23, 45-45 (1990)
Yukihiro Yamamoto et al.
Applied and environmental microbiology, 76(18), 6180-6185 (2010-08-03)
We specifically examined an exopeptidase, prolyl aminopeptidase (PAP), as a target for synthesis of proline-containing peptides. A PAP from Streptomyces thermoluteus subsp. fuscus NBRC14270 (PAP14270) was obtained using sequence-based screening. From PAP14270, 144Ser was replaced by Cys (scPAP14270) to give
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 364460-1G | |
| 364460-5G | 4061832641997 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service