347604
(3-Bromopropyl)trimethylammonium bromide
97%
Synonym(s):
3-Bromo-N,N,N-trimethylpropan-1-aminium Bromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Br(CH2)3N(CH3)3Br
CAS Number:
Molecular Weight:
261.00
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
210 °C (dec.) (lit.)
SMILES string
[Br-].C[N+](C)(C)CCCBr
InChI
1S/C6H15BrN.BrH/c1-8(2,3)6-4-5-7;/h4-6H2,1-3H3;1H/q+1;/p-1
InChI key
NNZGNZHUGJAKKT-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(3-Bromopropyl)trimethylammonium bromide is a quaternary ammonium salt and has various applications in organic chemistry, including the synthesis of hydrophilic-hydrophobic block copolymers. Additionally, this compound can be used as a surfactant in the synthesis of ultrathin nano scrolls (NSs).
Application
(3-Bromopropyl)trimethylammonium bromide can be used as a reagent in the synthesis of:
- Octa-cationic imidazolyl Al(III) phthalocyanine bifunctional catalyst CAT 2.
- Divalent surfactants like N-(3-trimethylammoniumpropyl)hexadecyldimethylammonium dibromide and N-(3-trimethylammoniumpropyl)octadecylammonium dibromide by reacting with surfactant precursor compounds hexadecyldimethylamine (C16NMe2), octadecyldimethylamine (C18NMe2) respectively.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Okazaki et al.
Analytical biochemistry, 145(1), 87-90 (1985-02-15)
Reduced lysozyme was alkylated with 3-bromopropyltrimethylammonium bromide to give reduced and S-3-(trimethylated amino)propylated lysozyme. It was soluble in a wide range of pH and is suitable as the protein substrate to determine protease specificity.
Damien E Dobson et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 19(1), 56-65 (2019-12-12)
A series of Cy5.5 dye analogs and targeted probes with net charges varied from -3 to 0 were synthesized by an optimized method, followed by comparing their spectral and photostability properties in saturated solutions of air, oxygen, and argon. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service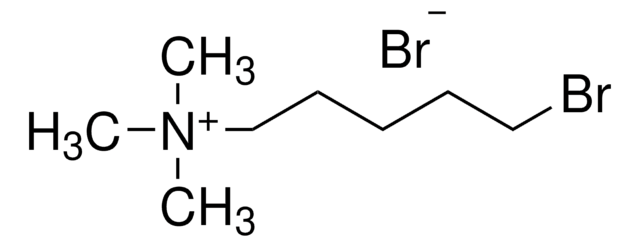




![N-[(3-(Anilinomethylene)-2-chloro-1-cyclohexen-1-yl)methylene]aniline monohydrochloride 94%](/deepweb/assets/sigmaaldrich/product/structures/779/226/f13a515f-1e57-497c-9450-4729b6b5cb79/640/f13a515f-1e57-497c-9450-4729b6b5cb79.png)