280704
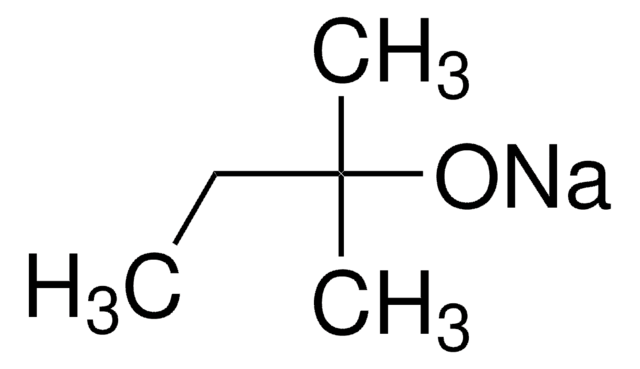
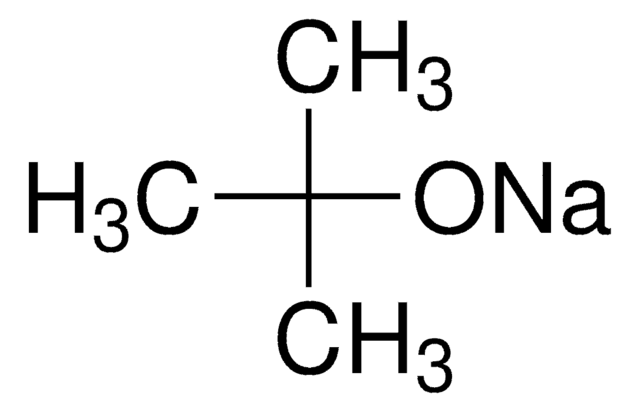
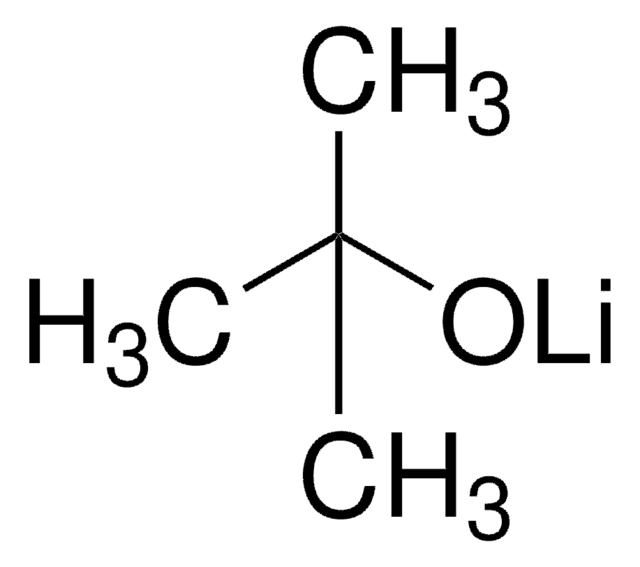
Sodium tert-pentoxide
95%
Synonym(s):
NaOt-Am, tert-Amyl alcohol sodium salt, Sodium tert-amoxide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NaOC(CH3)2CH2CH3
CAS Number:
Molecular Weight:
110.13
Beilstein:
3983987
EC Number:
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
SMILES string
[Na+].CCC(C)(C)[O-]
InChI
1S/C5H11O.Na/c1-4-5(2,3)6;/h4H2,1-3H3;/q-1;+1
InChI key
CGRKYEALWSRNJS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Sodium tert-pentoxide (NaOt-Am) is a commonly used base in the deoxycyanamidation of alcohols and in the N-cyanation of secondary amines. It can also be used in the preparation of complex reducing agents to reduce 1-halonaphthalenes and substituted halobenzenes to corresponding naphthalenes and benzenes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Deoxycyanamidation of Alcohols with N-Cyano-N-phenyl-p-methylbenzenesulfonamide (NCTS).
Ayres J N, et al.
Organic Letters, 19(14), 3835-3838 (2017)
N-Cyanation of Secondary Amines Using Trichloroacetonitrile.
Ayres J N, et al.
Organic Letters, 18(21), 5528-5531 (2016)
Activation of reducing agents: Sodium hydride-containing ?complex reducing agents?: I. Reduction of aromatic halides.
Guillaumet G, et al.
Journal of Organometallic Chemistry, 92(1), 43-47 (1975)
Brandon K Tate et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(28), 10160-10169 (2015-06-11)
Alkoxide-bridged disilver cations react with dihydrogen to form hydride-bridged cations, releasing free alcohol. Hydrogenolysis of neutral silver fluorides affords hydride-bridged disilver cations as their bifluoride salts. These reactions proceed most efficiently when the supporting ligands are expanded N-heterocyclic carbenes (NHCs)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service