277762
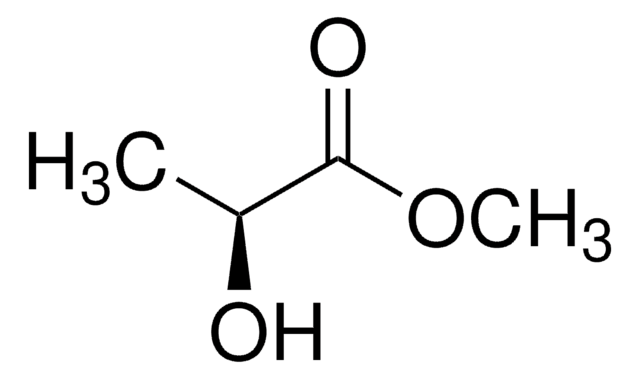
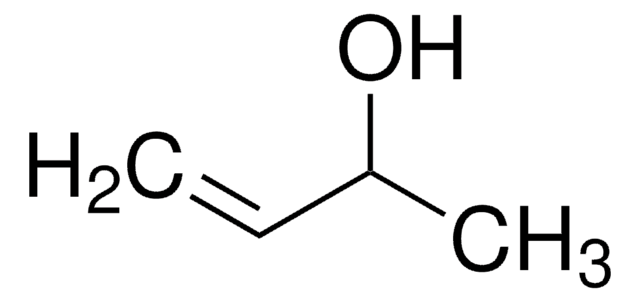
(+)-Methyl D-lactate
98%, optical purity ee: 96% (GLC)
Synonym(s):
D-Lactic acid methyl ester, Methyl (R)-(+)-lactate
About This Item
Recommended Products
Quality Level
Assay
98%
form
liquid
optical activity
[α]21/D +8.4°, neat
optical purity
ee: 96% (GLC)
greener alternative product characteristics
Safer Solvents and Auxiliaries
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.413 (lit.)
bp
144-145 °C (lit.)
density
1.09 g/mL at 25 °C (lit.)
functional group
ester
hydroxyl
greener alternative category
, Aligned
SMILES string
COC(=O)[C@@H](C)O
InChI
1S/C4H8O3/c1-3(5)4(6)7-2/h3,5H,1-2H3/t3-/m1/s1
InChI key
LPEKGGXMPWTOCB-GSVOUGTGSA-N
General description
Application
- Neomethymycin, a macrolide which contains 12-membered macrolactone that is used as a potent biological agent.
- PM-toxin A.
It can also be used as a chiral pool along with D-(-)-tartaric acid in the stereoselective total synthesis of separacenes A and B via Trost-Rychnovsky alkyne rearrangement, Horner-Wadsworth-Emmons olefination as well as Corey-Bakshi-Shibata reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
120.2 °F - closed cup
Flash Point(C)
49 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service