230197
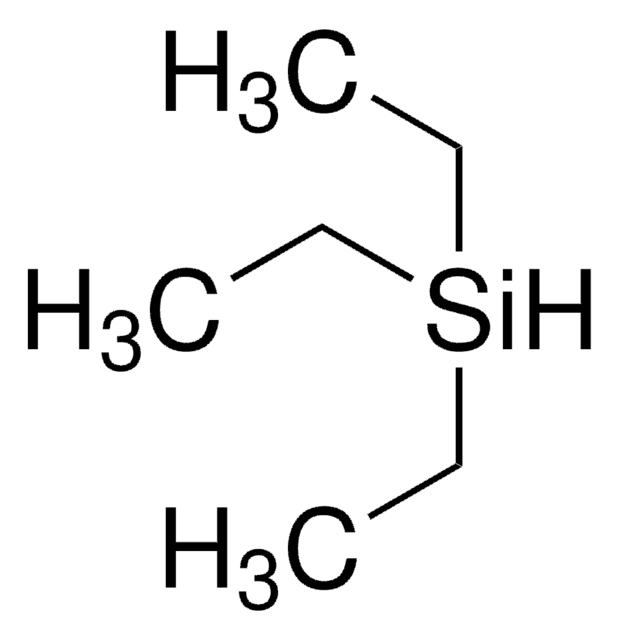
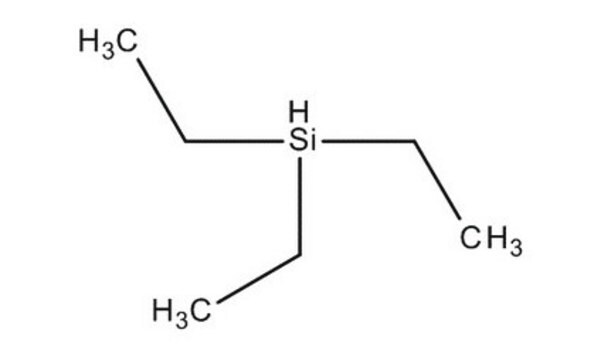
Triethylsilane
99%
Synonym(s):
NSC 93579, Triethylhydrosilane, Triethylsilicon hydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)3SiH
CAS Number:
Molecular Weight:
116.28
Beilstein:
1098278
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
reaction suitability
reagent type: reductant
refractive index
n20/D 1.412 (lit.)
bp
107-108 °C (lit.)
density
0.728 g/mL at 25 °C (lit.)
SMILES string
CC[SiH](CC)CC
InChI
1S/C6H16Si/c1-4-7(5-2)6-3/h7H,4-6H2,1-3H3
InChI key
AQRLNPVMDITEJU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Catalyst for:
Catalyst reactivation after catalyst polymerization of styrene
Studies involving the prediction of organosilicon flash points
- Synthesis of a spiro-oxindole blocker of Nav1.7 for the treatment of pain
- Redox initiated cationic polymerization
- Beckmann rearrangement of cyclododecanone oxime
- Regioselective reductive coupling of enones and allenes
Catalyst reactivation after catalyst polymerization of styrene
Studies involving the prediction of organosilicon flash points
Used in a study of the reduction of 2-chromanols; syn-selectivity observed with TES.
Versatile reducing agent
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
21.2 °F
Flash Point(C)
-6 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kelin Li et al.
Organic letters, 8(21), 4711-4714 (2006-10-06)
[reaction: see text] Reduction of C5-substituted 2-hydroxychromans selectively provides 2,4-cis-chromans using large silane reductants and 2,4-trans-chromans using the smaller silane PhSiH(3). The stereochemical outcome has been rationalized on the basis of a Curtin-Hammett kinetic situation arising from hydride delivery to
Zaihui Zhang et al.
Journal of medicinal chemistry, 56(2), 568-583 (2012-12-19)
Stearoyl-CoA desaturase-1 (SCD1) catalyzes de novo synthesis of monounsaturated fatty acids from saturated fatty acids. Studies have demonstrated that rodents lacking a functional SCD1 gene have an improved metabolic profile, including reduced weight gain, lower triglycerides, and improved insulin response.
Sultan Chowdhury et al.
Bioorganic & medicinal chemistry letters, 21(12), 3676-3681 (2011-05-17)
Starting from the oxindole 2a identified through a high-throughput screening campaign, a series of Na(V)1.7 blockers were developed. Following the elimination of undesirable structural features, preliminary optimization of the oxindole C-3 and N-1 substituents afforded the simplified analogue 9b, which
Emma J Ste Marie et al.
Journal of peptide science : an official publication of the European Peptide Society, 24(11), e3130-e3130 (2018-10-26)
Triisopropylsilane (TIS), a hindered hydrosilane, has long been utilized as a cation scavenger for the removal of amino acid protecting groups during peptide synthesis. However, its ability to actively remove S-protecting groups by serving as a reductant has largely been
Meital Bloch et al.
International journal of pharmaceutics, 478(2), 504-516 (2014-12-02)
To increase colonoscopy capability to discriminate benign from malignant polyps, we suggest combining two imaging approaches based on targeted polymeric platforms. Water-soluble cationized polyacrylamide (CPAA) was tagged with the near infrared (NIR) dye IR-783-S-Ph-COOH to form Flu-CPAA. The recognition peptide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service