224502
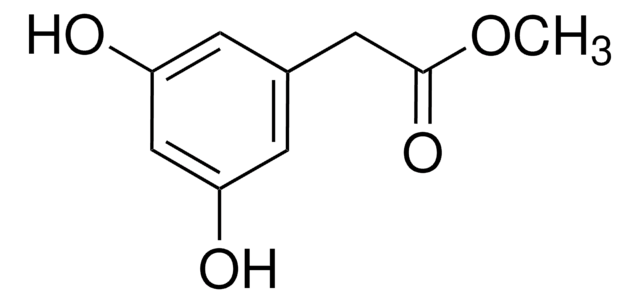
Methyl 4-hydroxyphenylacetate
ReagentPlus®, 99%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H4CH2CO2CH3
CAS Number:
Molecular Weight:
166.17
Beilstein:
2087538
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
solid
bp
162-163 °C/5 mmHg (lit.)
mp
55-58 °C (lit.)
functional group
ester
SMILES string
COC(=O)Cc1ccc(O)cc1
InChI
1S/C9H10O3/c1-12-9(11)6-7-2-4-8(10)5-3-7/h2-5,10H,6H2,1H3
InChI key
XGDZEDRBLVIUMX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyl 4-hydroxyphenylacetate inhibits the activity of tobacco mosaic virus (TMV).
Application
Methyl 4-hydroxyphenylacetate was used in the synthesis of 4-(2-hydroxy-3-isopropylamino)propoxyphenylacetic acid.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S H Jung et al.
Archives of pharmacal research, 23(3), 226-229 (2000-07-15)
To obtain the standard compounds of metoprolol for a pharmacokinetic study, a convenient synthetic procedure to prepare enantiomers of metoprolol (3a) and its major metabolites, 2-4-(2-hydroxy-3-isopropylamino)propoxyphenylethanol (3b) and 4-(2-hydroxy-3-isopropylamino)propoxyphenylacetic acid (4), was developed from their respective starting materials, 4-(2-methoxyethyl)phenol (1a)
Shuo Shen et al.
Natural product research, 27(24), 2286-2291 (2013-08-22)
A new compound 2-(4-hydroxybenzoyl) quinazolin-4(3H)-one (1) and four known compounds were isolated from a marine fungus Penicillium oxalicum 0312F1. The structure of the new compound 1 was elucidated based on the spectroscopic analysis. Bioactivity assays showed that 2-(4-hydroxybenzyl) quinazolin-4(3H)-one (2)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service