All Photos(1)
About This Item
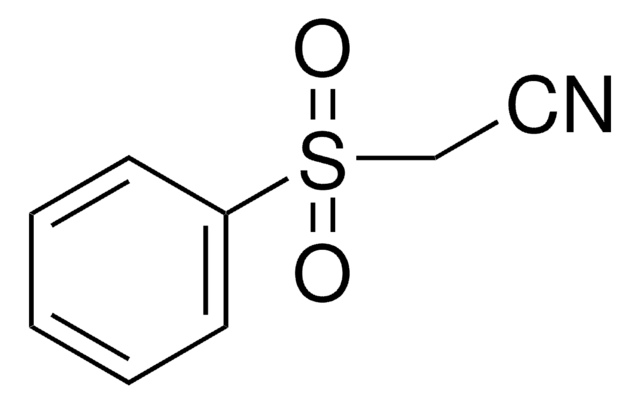
Linear Formula:
CH3SO2CH2CN
CAS Number:
Molecular Weight:
119.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
81-84 °C (lit.)
functional group
nitrile
sulfone
SMILES string
CS(=O)(=O)CC#N
InChI
1S/C3H5NO2S/c1-7(5,6)3-2-4/h3H2,1H3
InChI key
FOTRKCAZUSJCQD-UHFFFAOYSA-N
Related Categories
Application
(Methylsulfonyl)acetonitrile was used in the synthesis of:
- substituted δ-pyrone ring analogues
- 4,9-dihydropyrrolo[2,1-b]quinazolines containing electron withdrawing groups at the 3-position
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An Intramolecular N-Arylation Approach to 3-Functionalized 4, 9-Dihydropyrrolo [2, 1-b] quinazolines.
Suthiwangcharoen N, et al.
Journal of Heterocyclic Chemistry, 48(3), 706-709 (2011)
A novel entry to substituted chromones and furochromones through cyclopropane intermediates.
Gammill RB, et al.
Tetrahedron Letters, 33(8), 997-1000 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service