175560
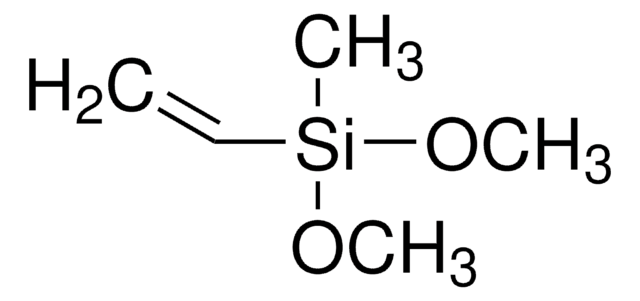
Triethoxyvinylsilane
97%
Synonym(s):
(Triethoxysilyl)ethylene, Vinyltriethoxysilane
About This Item
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.398 (lit.)
bp
160-161 °C (lit.)
62-63 °C/20 mmHg (lit.)
density
0.903 g/mL at 25 °C (lit.)
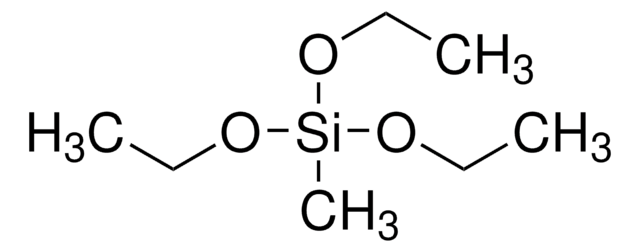
SMILES string
CCO[Si](OCC)(OCC)C=C
InChI
1S/C8H18O3Si/c1-5-9-12(8-4,10-6-2)11-7-3/h8H,4-7H2,1-3H3
InChI key
FWDBOZPQNFPOLF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
93.2 °F - closed cup
Flash Point(C)
34 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
A viable alternative to the popular Stille and Suzuki coupling reactions mainly due to the formation of nontoxic byproducts and stability to many reaction conditions.
Over the past several years, Pd-catalyzed cross-coupling of silicon compounds has rapidly gained acceptance as a suitable alternative to more commonly known methods such as: Stille (Sn), Kumada (Mg), Suzuki (B), and Negishi (Zn) cross-couplings.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service