All Photos(1)
About This Item
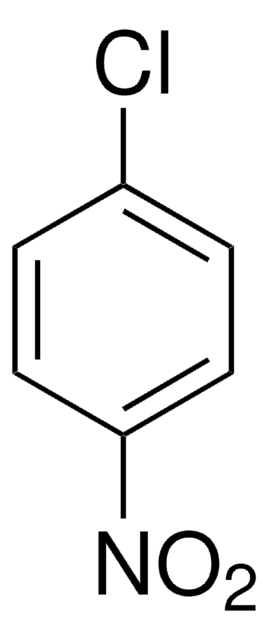
Linear Formula:
ClC6H4OCH3
CAS Number:
Molecular Weight:
142.58
Beilstein:
1858901
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.535 (lit.)
bp
198-202 °C (lit.)
mp
−18 °C (lit.)
density
1.164 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(Cl)cc1
InChI
1S/C7H7ClO/c1-9-7-4-2-6(8)3-5-7/h2-5H,1H3
InChI key
YRGAYAGBVIXNAQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Chloroanisole is an electron-rich chloroarene. It undergoes Ullmann-type homocoupling catalyzed by in situ generated Pd colloids. Reaction of 4-chloroanisole with Cu(II)-smectite in n-hexane, carbon tetrachloride or dichloromethane has been studied. The nucleophilic photosubstitution reactions, photocyanation and photohydrolysis of 4-chloroanisole has been studied by time resolved spectroscopy.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antonio Monopoli et al.
The Journal of organic chemistry, 75(11), 3908-3911 (2010-05-14)
An efficient and highly sustainable Ullmann-type homocoupling of bromo- and chloroarenes, including the more challenging electron-rich chloroarenes (e.g., 4-chloroanisole), catalyzed by in situ generated Pd colloids, is carried out in aqueous medium under relatively mild conditions (temperatures ranging from 40
Laser spectroscopic study of the nucleophilic photosubstitution of 4-chloroanisole and 4-fluoroanisole in aqueous solutions.
Lemmetyinen H, et al.
Journal of Photochemistry, 30(3), 315-338 (1985)
Single electron transfer mechanism of oxidative dechlorination of 4-chloroanisole on copper (II)-smectite.
Govindaraj N, et al.
Environmental Science & Technology, 21(11), 1119-1123 (1987)
Qingqing Mei et al.
Science advances, 4(5), eaaq0266-eaaq0266 (2018-05-26)
Ether bond activation is very interesting because the synthesis of many valuable compounds involves conversion of ethers. Moreover, C-O bond cleavage is also very important for the transformation of biomass, especially lignin, which abundantly contains ether bonds. Developing efficient methods
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service