154210
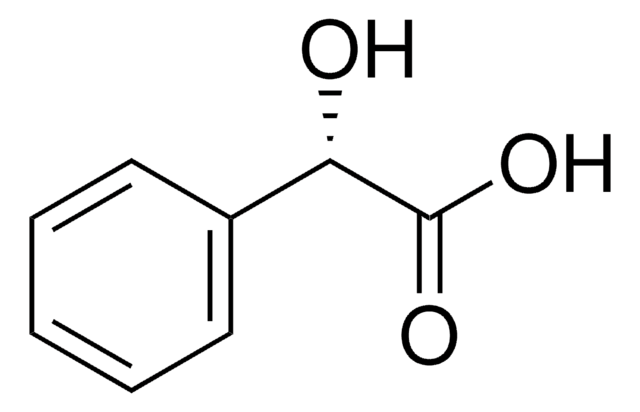
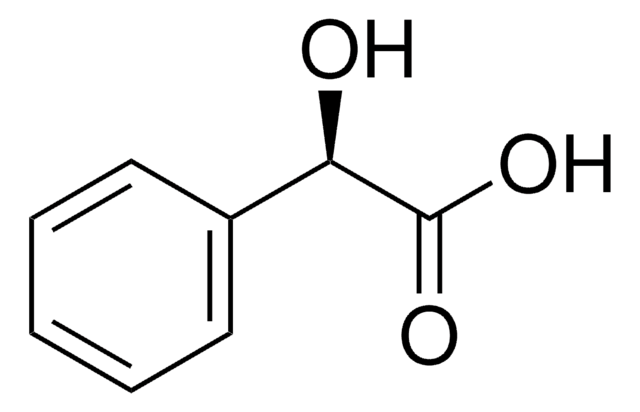
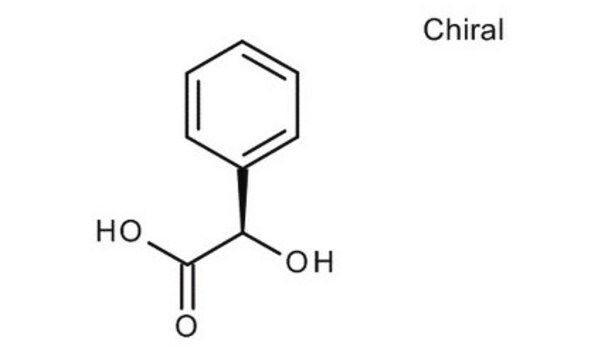
(R)-(−)-Mandelic acid
ReagentPlus®, ≥99%
Synonym(s):
(R)-α-Hydroxyphenylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(OH)CO2H
CAS Number:
Molecular Weight:
152.15
Beilstein:
2691094
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99%
form
solid
optical activity
[α]23/D −153°, c = 2.5 in H2O
mp
131-133 °C (lit.)
SMILES string
O[C@@H](C(O)=O)c1ccccc1
InChI
1S/C8H8O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7,9H,(H,10,11)/t7-/m1/s1
InChI key
IWYDHOAUDWTVEP-SSDOTTSWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(R)-(-)-Mandelic acid, a chiral resolving agent, is also used as a building block to synthesize pharmaceutical drugs such as penicillin and cephalosporin. It can be synthesized from (R,S)-mandelonitrile with high yield and enantioselectivity using nitrilase enzyme .
(R)-(−)-Mandelic acid is a carboxylic acid used as a starting material to synthesize antibiotics, antitumor agents, and antiobesity drugs.
(R)-(−)-Mandelic acid is a carboxylic acid used as a starting material to synthesize antibiotics, antitumor agents, and antiobesity drugs.
Application
(R)-(-)-Mandelic acid has been used in studies to assess its ability to undergo spontaneous oscillatory chiral conversion and spontaneous condensation to form polymandelic acid.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enzyme-catalysed optical resolution of mandelic acid via RS (?)-methyl mandelate in non-aqueous media.
Yadav GD and Sivakumar P.
Biochemical Engineering Journal, 19(2), 101-107 (2004)
TLC in a search for structural limitations of spontaneous oscillatory in-vitro chiral conversion. a-hydroxybutyric and mandelic acids.
Sajewicz M, et al.
J. Planar Chromatogr., 22(4), 241-248 (2009)
Production of R-(-)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750.
Yamamoto K, et al.
Applied and Environmental Microbiology, 57(10), 3028-3032 (1991)
On the spontaneous condensation of selected hydroxy acids.
Sajewicz M, et al.
Acta Chromatographica , 21(2), 259-271 (2009)
Ya-Ping Xue et al.
Journal of industrial microbiology & biotechnology, 38(2), 337-345 (2010-07-20)
(R)-(-)-Mandelic acid (R-MA) is an important intermediate with broad uses. Recently, R-MA production using nitrilase has been gaining more and more attention due to its higher productivity and enantioselectivity. In this work, a new bacterium WT10, which exhibited favorable nitrilase
Chromatograms
suitable for GCsuitable for GCGlobal Trade Item Number
| SKU | GTIN |
|---|---|
| 154210-5KG | |
| 154210-10KG | |
| 154210-25G | 4061838741332 |
| 154210-500G | |
| 154210-5G | 4061838741349 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service