All Photos(2)
About This Item
Empirical Formula (Hill Notation):
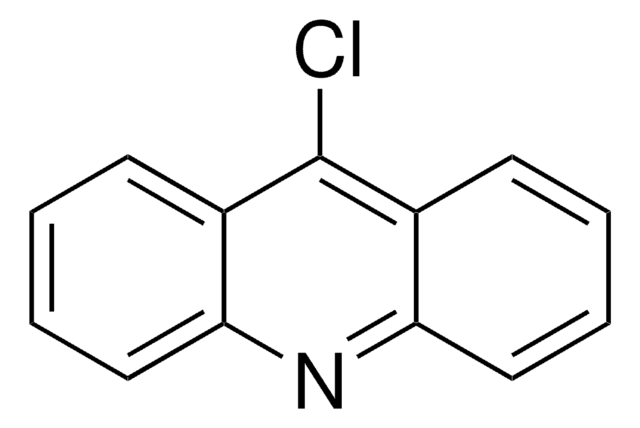
C14H9Cl2NO
CAS Number:
Molecular Weight:
278.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
163-165 °C (lit.)
functional group
chloro
SMILES string
COc1ccc2nc3cc(Cl)ccc3c(Cl)c2c1
InChI
1S/C14H9Cl2NO/c1-18-9-3-5-12-11(7-9)14(16)10-4-2-8(15)6-13(10)17-12/h2-7H,1H3
InChI key
RYRNQWYNHLLOGX-UHFFFAOYSA-N
General description
6,9-Dichloro-2-methoxyacridine on reaction with quinolizidinylalkylamines yields 4-aminoquinoline and 9-aminoacridine derivatives.
Application
6,9-Dichloro-2-methoxyacridine was used in the synthesis of 9-amino-6-chloro-2-methoxyacridine, N′-(6-Chloro-2-methoxy-acridin-9-yl)-heptylamine and N,N′-bis-(6-chloro-2-methoxy-acridin-9-yl)-hexane-1,6-diamine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Origin of the complex fluorescence emission of 9-amino-6-chloro-2-methoxyacridine. 1. Experiment.
Fan P, et al.
The Journal of Physical Chemistry, 93(18), 6615-6622 (1989)
C Boido Canu et al.
Bollettino chimico farmaceutico, 128(6), 212-215 (1989-06-01)
By reacting three quinolizidinylalkylamines with 4,7-dichloroquinoline and 6,9-dichloro-2-methoxyacridine six derivatives of 4-aminoquinoline and 9-aminoacridine were obtained. These compounds, which are of interest as potential antibacterial, antiprotozoarian, anti-helminthic and antitumoral agents, so far have been tested against lymphocytic leukemia P 388
Lucie Guetzoyan et al.
Bioorganic & medicinal chemistry, 17(23), 8032-8039 (2009-11-03)
A series of acridine derivatives were synthesised and their in vitro antimalarial activity was evaluated against one chloroquine-susceptible strain (3D7) and three chloroquine-resistant strains (W2, Bre1 and FCR3) of Plasmodium falciparum. Structure-activity relationship showed that two positives charges as well
Ana Gomes et al.
ChemMedChem, 9(2), 305-310 (2014-01-30)
Plasmodium falciparum, the causative agent of the most lethal form of malaria, is becoming increasingly resistant to most available drugs. A convenient approach to combat parasite resistance is the development of analogues of classical antimalarial agents, appropriately modified in order
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 145688-25G | 4061838736307 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service