13454
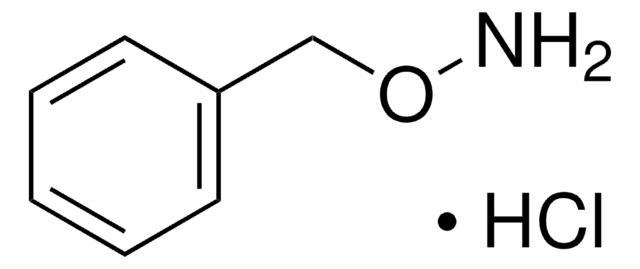
N-Benzylhydroxylamine hydrochloride
puriss., ≥99.0% (AT)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2NHOH · HCl
CAS Number:
Molecular Weight:
159.61
Beilstein:
507948
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
puriss.
Quality Level
Assay
≥99.0% (AT)
form
solid
mp
~105 °C
108-110 °C (lit.)
functional group
amine
phenyl
SMILES string
Cl.ONCc1ccccc1
InChI
1S/C7H9NO.ClH/c9-8-6-7-4-2-1-3-5-7;/h1-5,8-9H,6H2;1H
InChI key
YSNXOQGDHGUKCZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N-Benzylhydroxylamine hydrochloride was used in the synthesis of sugar derived nitrones. It was used as starting reagent in the synthesis of fluoro isoxazoline and isoxazolidine derivatives using flouro nitrone.
Biochem/physiol Actions
N-Benzylhydroxylamine is a potential pharmacological agent in the prevention and progression of acrolein-induced damage to the retinal pigment epithelium.
Other Notes
Formation of nitrones from carbonyl compounds and [2+3]-cycloaddition with olefins to isoxazolidines, which are versatile intermediates; Pd-catalyzed hydroxylamination of allylic esters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R Herrera et al.
The Journal of organic chemistry, 66(4), 1252-1263 (2001-04-21)
Captodative olefins 1-acetylvinyl carboxylates proved to be highly regioselective dipolarophiles in 1,3-dipolar cycloadditon to propionitrile oxide, arylphenylnitrile imines, diazoalkanes, and nitrones to yield the corresponding 5-substituted heterocycles. The addition of the latter was also stereoselective, being slightly susceptible to steric
B.J. Wakefield
Sci. Synth., 11, 229-229 (2002)
Ionic liquid mediated synthesis of some novel fluoro isoxazolidine and isoxazoline derivatives using N-benzyl fluoro nitrone via cycloaddition reaction and their antimicrobial activities.
Chakraborty B, et al.
Indian J. Chem. B, 52(10), 1342-1351 (2013)
T. Kawakami et al.
Bulletin of the Chemical Society of Japan, 73, 2423-2423 (2000)
J.E. Baldwin et al.
Tetrahedron, 42, 3097-3097 (1986)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service