All Photos(3)
About This Item
Empirical Formula (Hill Notation):
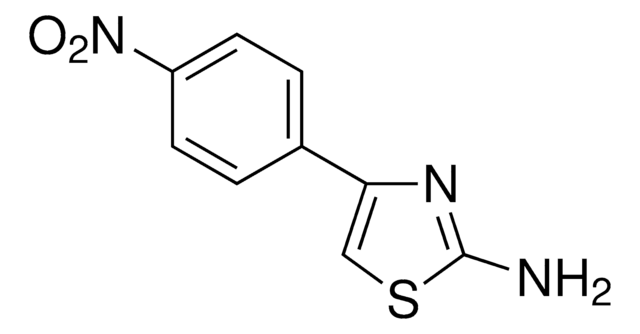
C3H3N3O2S
CAS Number:
Molecular Weight:
145.14
Beilstein:
126797
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
195-200 °C (dec.) (lit.)
solubility
95% ethanol: soluble 1g/150g at 20 °C
diethyl ether: soluble 1g/250g at 20 °C
chloroform: insoluble
water: slightly soluble
functional group
nitro
SMILES string
Nc1ncc(s1)[N+]([O-])=O
InChI
1S/C3H3N3O2S/c4-3-5-1-2(9-3)6(7)8/h1H,(H2,4,5)
InChI key
MIHADVKEHAFNPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-5-nitrothiazole(ANT) is a hypoxic radiosensitizing drug. ANT forms square-planar complex, trans-[PdCl2(ANT)2] with palladium in methanol.
Application
2-Amino-5-nitrothiazole was used as diazo component in the synthesis of monoazo disperse dyes. It was used as matrix during matrix-assisted laser desorption/ionization time-of-flight mass spectrometric study of oligonucleotide and protein.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reaction of 2-amino-5-nitrothiazole with palladium salts.
Farrell N and Carneiro TM.
Inorgorganica Chimica Acta, 126(2), 137-139 (1987)
Analysis of Oligonucleotide and Protein via Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry Using 2-Amino-5-nitrothiazole as an Effective Matrix [J].
ZHOU LH, et al.
Chemical Research in Chinese Universities, 12, 012-012 (2004)
Synthesis and Spectral Properties of Hetaryl Monoazo dyes derived from 2-amino-5-nitrothiazole.
OTUTU JO, et al.
Orient. J. Chem., 27(4), 2011-2011 (1389)
Evaluation of 2-amino-5-nitrothiazole as a hypoxic cell radiosensitizer.
S Rockwell et al.
Radiation research, 90(3), 575-585 (1982-06-01)
T Eric Ballard et al.
Bioorganic & medicinal chemistry letters, 20(12), 3537-3539 (2010-05-22)
Head group analogues of the antibacterial and antiparasitic drug nitazoxanide (NTZ) are presented. A library of 39 analogues was synthesized and assayed for their ability to suppress growth of Helicobacter pylori, Campylobacter jejuni, Clostridium difficile and inhibit NTZ target pyruvate:ferredoxin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service