128376
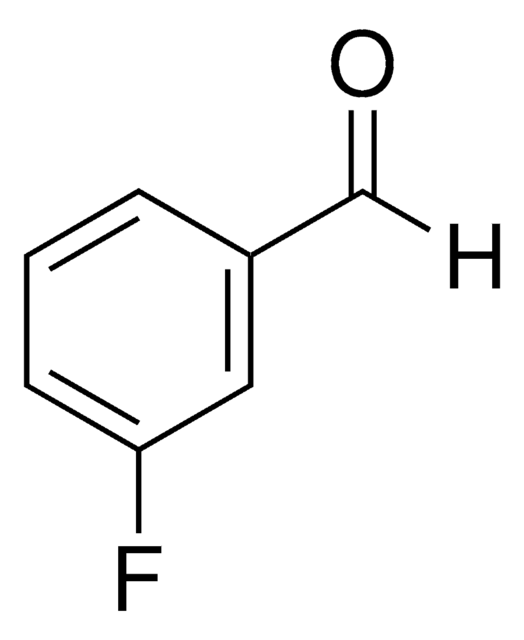
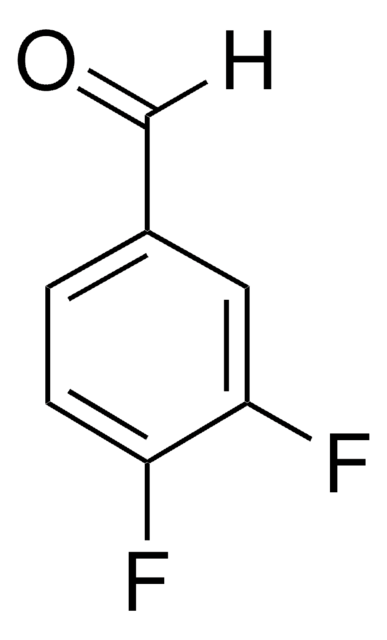
4-Fluorobenzaldehyde
98%
Synonym(s):
p-Fluorobenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
FC6H4CHO
CAS Number:
Molecular Weight:
124.11
Beilstein:
385857
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.521 (lit.)
bp
181 °C/758 mmHg (lit.)
mp
−10 °C (lit.)
density
1.157 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(F)cc1
InChI
1S/C7H5FO/c8-7-3-1-6(5-9)2-4-7/h1-5H
InChI key
UOQXIWFBQSVDPP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Fluorobenzaldehyde was used in the preparation of pyrazolopyridine UR-13756.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
132.8 °F - closed cup
Flash Point(C)
56 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Abdul Hafeez et al.
Polymers, 11(9) (2019-09-22)
Bis-aldehyde monomers 4-(4'-formyl-phenoxy)benzaldehyde (3a), 3-methoxy-4-(4'-formyl-phenoxy)benzaldehyde (3b), and 3-ethoxy-4-(4'-formyl-phenoxy)benzaldehyde (3c) were synthesized by etherification of 4-fluorobenzaldehyde (1) with 4-hydroxybenzaldehyde (2a), 3-methoxy-4-hydroxybenzaldehyde (2b), and 3-ethoxy-4-hydroxybenzaldehyde (2c), respectively. Each monomer was polymerized with p-phenylenediamine and 4,4'-diaminodiphenyl ether to yield six poly(azomethine)s. Single crystal
Mark C Bagley et al.
Future medicinal chemistry, 2(2), 193-201 (2011-03-24)
UR-13756 is a potent and selective p38 mitogen-activated protein kinase (MAPK) inhibitor, reported to have good bioavailability and pharmacokinetic properties and, thus, is of potential use in the treatment of accelerated aging in Werner syndrome. Irradiation of 2-chloroacrylonitrile and methylhydrazine
Xuemei Tian et al.
Enzyme and microbial technology, 84, 32-40 (2016-02-02)
The first Novozym 435 lipase-catalyzed Morita-Baylis-Hillman (MBH) reaction with amides as co-catalyst was realized. Results showed that neither Novozym 435 nor amide can independently catalyze the reaction. This co-catalytic system that used a catalytic amount of Novozym 435 with a
Ana Margarida Araújo et al.
Archives of toxicology, 92(11), 3307-3323 (2018-09-27)
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) is a well-known hepatotoxic drug. Although its toxicity has been thoroughly studied at high concentrations, there is still insufficient knowledge on possible alterations of cell function at subtoxic concentrations, which are in fact more representative concentrations of
Bin-Yu Wen et al.
Journal of Asian natural products research, 21(7), 702-715 (2019-01-01)
Desmosdumotin C (Des C), a natural product isolated from the roots of Desmos dumosus, has shown good antitumor activity. A three dimensional quantitative structure-activity relationship (QSAR) study using the comparative molecular field analysis (CoMFA) method was performed on 32 Des
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service