All Photos(1)
About This Item
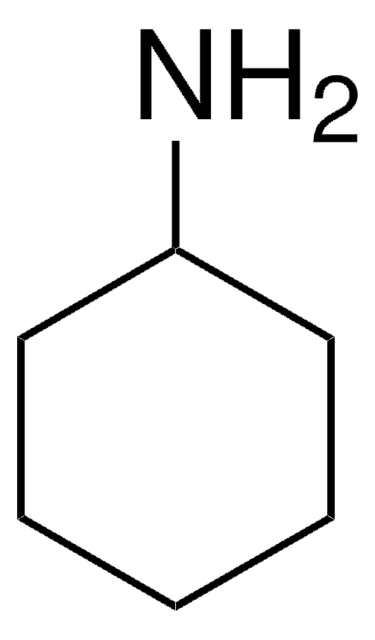
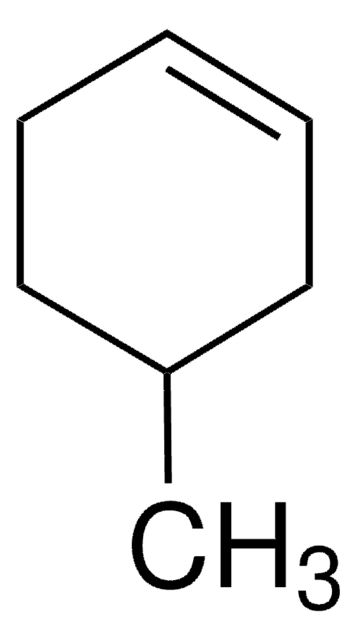
Linear Formula:
HCONHC6H11
CAS Number:
Molecular Weight:
127.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
113 °C/700 mmHg (lit.)
mp
36-41 °C (lit.)
functional group
amide
SMILES string
O=CNC1CCCCC1
InChI
1S/C7H13NO/c9-6-8-7-4-2-1-3-5-7/h6-7H,1-5H2,(H,8,9)
InChI key
SWGXDLRCJNEEGZ-UHFFFAOYSA-N
Gene Information
human ... ADH1A(124) , ADH1B(125) , ADH1C(126) , ADH4(127) , ADH7(131) , EPHX2(2053)
mouse ... Ephx2(13850)
General description
N-Cyclohexylformamide binds to the complex of horse liver alcohol dehydrogenase with NADH and is similar to the Michaelis complex for aldehyde reduction catalyzed by the enzyme. It is formed during hydration of cyclohexyl isocyanide catalyzed by a novel enzyme isonitrile hydratase from Pseudomonas putida N19-2 .
Application
N-Cyclohexylformamide was used in the synthesis of 2-methylidene-1,3-selenazolidine derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
>235.4 °F - closed cup
Flash Point(C)
> 113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selenium-containing heterocycles from isoselenocyanates: synthesis of 2-methylidene-1, 3-selenazolidine derivatives.
Sommen GL, et al.
Tetrahedron, 62(14), 3344-3354 (2006)
Discovery of a novel enzyme, isonitrile hydratase, involved in nitrogen-carbon triple bond cleavage.
M Goda et al.
The Journal of biological chemistry, 276(26), 23480-23485 (2001-04-18)
Isonitrile containing an N triple bond C triple bond was degraded by microorganism sp. N19-2, which was isolated from soil through a 2-month acclimatization culture in the presence of this compound. The isonitrile-degrading microorganism was identified as Pseudomonas putida. The
H Deng et al.
Biochemistry, 37(40), 14267-14278 (1998-10-07)
The binding of N-cyclohexylformamide (CXF) to the complex of horse liver alcohol dehydrogenase with NADH mimics that of the Michaelis complex for aldehyde reduction catalyzed by the enzyme. The Raman spectra of bound CXF and its 13C- and 15N-substituted derivatives
Uncompetitive inhibitors of alcohol dehydrogenases.
B V Plapp et al.
Advances in experimental medicine and biology, 463, 295-303 (1999-06-03)
S Svensson et al.
Journal of molecular biology, 302(2), 441-453 (2000-09-06)
The structure of mouse class II alcohol dehydrogenase (ADH2) has been determined in a binary complex with the coenzyme NADH and in a ternary complex with both NADH and the inhibitor N-cyclohexylformamide to 2.2 A and 2.1 A resolution, respectively.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service