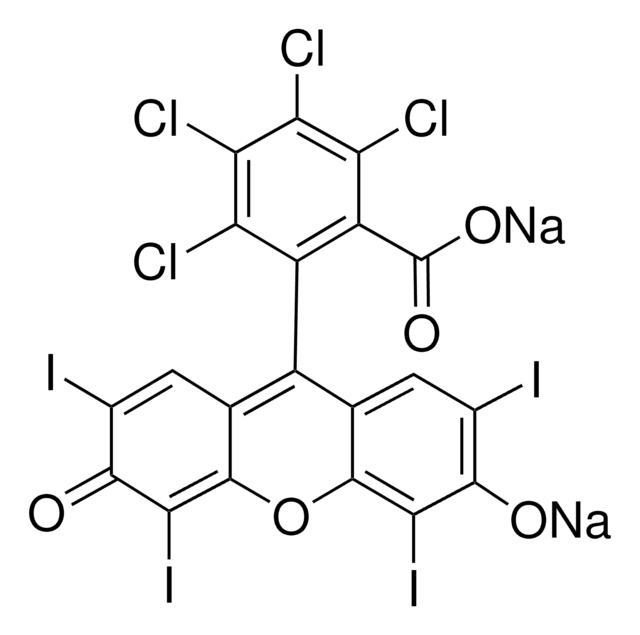
105481
1,3-Diphenylisobenzofuran
97%
Synonym(s):
1,3-Diphenyl-2-benzofuran, 2,5-Diphenyl-3,4-benzofuran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H14O
CAS Number:
Molecular Weight:
270.32
Beilstein:
199922
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
128-130 °C (lit.)
functional group
phenyl
SMILES string
c1ccc(cc1)-c2oc(-c3ccccc3)c4ccccc24
InChI
1S/C20H14O/c1-3-9-15(10-4-1)19-17-13-7-8-14-18(17)20(21-19)16-11-5-2-6-12-16/h1-14H
InChI key
ZKSVYBRJSMBDMV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,3-Diphenylisobenzofuran is a fluorescent dye.1,3-Diphenylisobenzofuran is the model compound in studies of singlet fission.
Application
1,3-Diphenylisobenzofuran(DPBF) was used as a fluorescent probe for detection of superoxide anion radical (O2−) inside the membrane lipid layer by DPBF fluorescence quenching method. 1,3-Diphenylisobenzofuran(DPBF) was used as quencher during the photoinactivation of TA-3 mouse mammary carcinoma cells containing hematoporphyrin. 1,3-Diphenylisobenzofuran(DPBF) was used to study the single crystal molecular structure and solution photophysical properties of DPBF.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Huanhuan Liu et al.
Scientific reports, 9(1), 8393-8393 (2019-06-12)
A great number of fluorescent probes have been developed for detecting singlet oxygen (1O2), which is considered to be one of the most effective reactive oxygen species (ROS), especially in clinical applications. The commercially available fluorescent probe Singlet Oxygen Sensor
Wenquan Ou et al.
Theranostics, 8(17), 4574-4590 (2018-10-04)
The efficacy of combined near-infrared (NIR) and immune therapies for inhibiting tumor growth and recurrence has gained increasing research attention. Regulatory T cells in the tumor microenvironment constitute a major obstacle in achieving robust CD8+ T cell antitumor immunotherapy. In
Zhihao Han et al.
Journal of biophotonics, 10(12), 1607-1616 (2017-01-21)
It is an emerging focus to explore controlled release drug delivery systems for simultaneous cancer imaging and therapy. Herein, we synthesized a photothermal sensitive multifunctional nano-liposome drug delivery system, with doxorubicin wrapped in the hydropholic layer as the therapeutical agent
Andrew F Schwerin et al.
The journal of physical chemistry. A, 114(3), 1457-1473 (2009-12-23)
Single crystal molecular structure and solution photophysical properties are reported for 1,3-diphenylisobenzofuran (1), of interest as a model compound in studies of singlet fission. For the ground state of 1 and of its radical cation (1(+*)) and anion (1(-*)), we
K R Weishaupt et al.
Cancer research, 36(7 PT 1), 2326-2329 (1976-07-01)
Singlet oxygen, a metastable state of normal triplet oxygen, has been identified as the cytotoxic agent that is probably responsible for in vitro inactivation of TA-3 mouse mammary carcinoma cells following incorporation of hematoporphyrin and exposure to red light. This
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service