1457607
USP
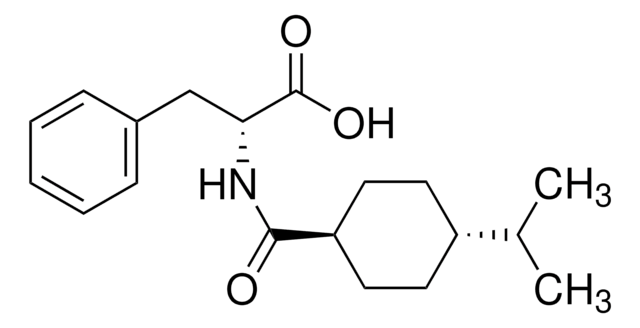
Nateglinide
United States Pharmacopeia (USP) Reference Standard
동의어(들):
Fastic, N-[(trans-4-Isopropylcyclohexyl)carbonyl]-D-phenylalanine, Starlix, Starsis
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C19H27NO3
CAS Number:
Molecular Weight:
317.42
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
nateglinide
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
CC(C)[C@@H]1CC[C@H](CC1)C(=O)N[C@H](Cc2ccccc2)C(O)=O
InChI
1S/C19H27NO3/c1-13(2)15-8-10-16(11-9-15)18(21)20-17(19(22)23)12-14-6-4-3-5-7-14/h3-7,13,15-17H,8-12H2,1-2H3,(H,20,21)(H,22,23)/t15-,16-,17-/m1/s1
InChI key
OELFLUMRDSZNSF-BRWVUGGUSA-N
유전자 정보
human ... ABCC8(6833) , KCNJ11(3767)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
생화학적/생리학적 작용
Nateglinide is a Kir6.2/SUR1 channel inhibitor and antidiabetic.
Nateglinide is a Kir6.2/SUR1 channel inhibitor and antidiabetic. It is selective for the SUR1 subtype, which is found on pancreatic islet cells. Nateglinide evokes KATP channel-dependent insulin secretion (50-200 μM) in the absence and presence of insulin.
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
[Effects of nateglinide in impaired glucose tolerance subjects].
Takahisa Hirose
Nihon rinsho. Japanese journal of clinical medicine, 63 Suppl 2, 438-443 (2005-03-23)
I W Campbell
International journal of clinical practice, 59(10), 1218-1228 (2005-09-24)
Therapy for type 2 diabetes mellitus should aim to control not only fasting, but also postprandial glucose levels. Nateglinide, a d-phenylalanine derivative, restores postprandial early phase insulin secretion in a transient and glucose-sensitive manner without affecting basal insulin levels. As
Marc K Israel et al.
Vascular health and risk management, 4(6), 1167-1178 (2008-01-01)
The increasing prevalence of type 2 diabetes provides impetus for both development of new drugs to improve glycemic control and for reconsideration of treatment strategies with existing agents. Combination therapy with complementary drug classes that act on different aspects of
T Ikenoue et al.
Nihon yakurigaku zasshi. Folia pharmacologica Japonica, 116(3), 171-180 (2000-10-14)
An early defect in Type 2 diabetes is the loss of acute insulin release after food intake, which causes prolonged elevation of postprandial glucose levels. Suppressing postprandial hyperglycemia is considered to be very important for preventing diabetic complications. Sulfonylureas are
L S Phillips et al.
International journal of clinical practice, 57(6), 535-541 (2003-08-16)
Nateglinide is a new oral antidiabetic agent that stimulates insulin release promptly after its pre-meal administration in a strongly glucose-dependent fashion. Because its insulinotropic effects are short in duration, nateglinide specifically targets postprandial hyperglycaemia with a low potential to elicit
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.