SML0212
PSB36
≥98% (HPLC)
동의어(들):
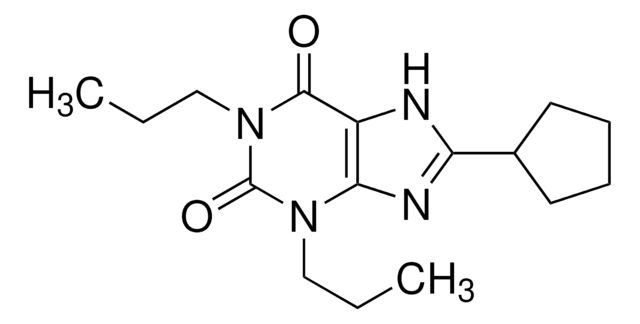
1-Butyl-3-(3-hydroxypropyl)-8-(3-noradamantyl)xanthine, 1-Butyl-8-(hexahydro-2,5-methanopentalen-3a(1H)-yl)-3,9-dihydro-3-(3-hydroxypropyl)-1H-purine-2,6-dione, PSB-36
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
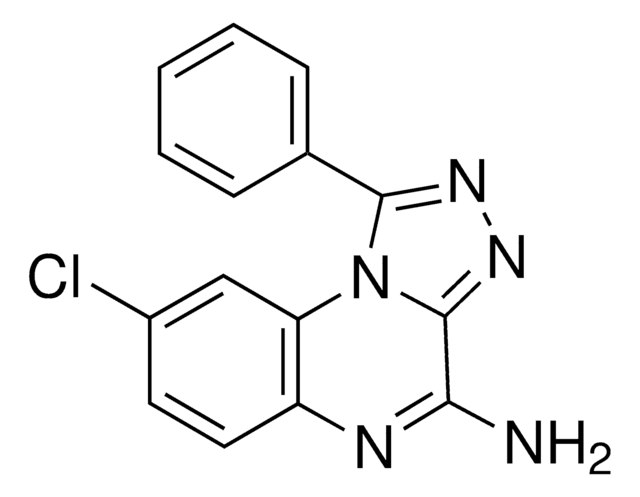
실험식(Hill 표기법):
C21H30N4O3
CAS Number:
Molecular Weight:
386.49
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
저장 조건
desiccated
색상
white to tan
solubility
DMSO: ≥20 mg/mL
저장 온도
2-8°C
SMILES string
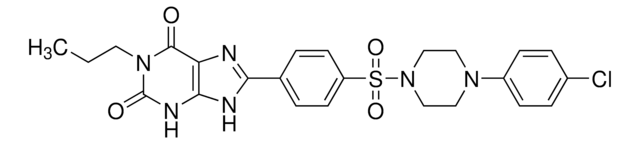
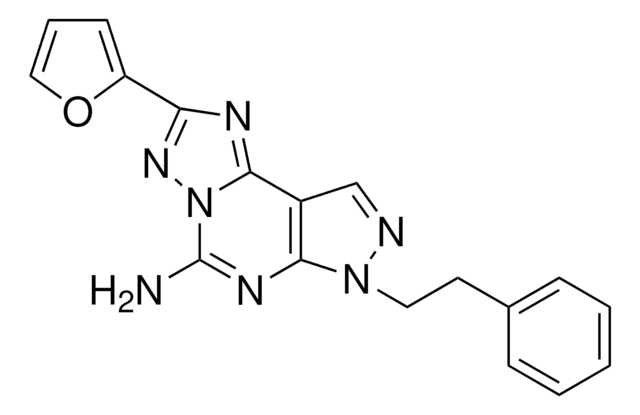
CCCCN1C(=O)N(CCCO)c2nc([nH]c2C1=O)C34C[C@@H]5C[C@@H](C[C@H]3C5)C4
InChI
1S/C21H30N4O3/c1-2-3-5-25-18(27)16-17(24(20(25)28)6-4-7-26)23-19(22-16)21-11-13-8-14(12-21)10-15(21)9-13/h13-15,26H,2-12H2,1H3,(H,22,23)/t13-,14+,15-,21-
InChI key
CIBIXJYFYPFMTN-FZUGUKJMSA-N
애플리케이션
PSB36 was used to examine the role of A1-adenosine receptor-mediated cell signaling in CD39 expression in pancreatic b-cells of streptozotocin-induced diabetic mice.
생화학적/생리학적 작용
Inhibition of A1 adenosine receptor by PSB36 modulates the spinal antinociception in animal models.
PSB36 is a very potent, selective antagonist of the adenosine A1 receptor. The compound selectivity (Ki) for human A1, A2A, A2B and A3 receptors is 0.7, 980, 187 and 2300 respectively. PSB36 is considerably more potent that DPCPX (EC50 0.012 nM vs 2.9 nM)
PSB36 is an adenosine A1 AR antagonist
특징 및 장점
This compound is featured on the Adenosine Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Yuta Tanaka et al.
Biological & pharmaceutical bulletin, 43(3), 516-525 (2019-12-24)
It is therapeutically important to elucidate the factors involved in the radiation resistance of tumors. We previously showed that ATP is released from mouse melanoma B16 cells in response to γ-irradiation, but the role of adenosine, a metabolite of ATP
Osama M Abo-Salem et al.
The Journal of pharmacology and experimental therapeutics, 308(1), 358-366 (2003-10-18)
Caffeine, an adenosine A1, A2A, and A2B receptor antagonist, is frequently used as an adjuvant analgesic in combination with nonsteroidal anti-inflammatory drugs or opioids. In this study, we have examined the effects of novel specific adenosine receptor antagonists in an
Siqi Chen et al.
Cancer immunology research, 8(8), 1064-1074 (2020-05-10)
Accumulating evidence suggests that inhibiting adenosine-generating ecto-enzymes (CD39 and CD73) and/or adenosine A2A or A2B receptors (R) stimulates antitumor immunity and limits tumor progression. Although activating A2ARs or A2BRs causes similar immunosuppressive and protumoral functions, few studies have investigated the
Luca Soattin et al.
Frontiers in physiology, 11, 493-493 (2020-07-01)
Adenosine leads to atrial action potential (AP) shortening through activation of adenosine 1 receptors (A1-R) and subsequent opening of G-protein-coupled inwardly rectifying K+ channels. Extracellular production of adenosine is drastically increased during stress and ischemia. The aim of this study
Kazuki Kitabatake et al.
Biological & pharmaceutical bulletin, 44(2), 197-210 (2020-12-04)
Glioblastoma is the most common malignant tumor of the central nervous system and is treated with a combination of surgery, radiation and chemotherapy. However, the tumor often acquires radiation resistance, which is characterized by an increased DNA damage response (DDR).
문서
We offers many products related to adenosine receptors for your research needs.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.