SAB4200812
Anti-HSP90 antibody produced in rabbit
affinity isolated antibody, buffered aqueous solution
동의어(들):
(LAP-2), HSP86, Heat shock 86 kDa, Heat shock protein HSP 90-alpha, LPS-associated protein 2, Lipopolysaccharide-associated protein 2, Renal carcinoma antigen NY-REN-38
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
UNSPSC 코드:
12352203
NACRES:
NA.41
추천 제품
생물학적 소스
rabbit
항체 형태
affinity isolated antibody
항체 생산 유형
primary antibodies
클론
polyclonal
양식
buffered aqueous solution
분자량
~90 kDa
종 반응성
human, mouse, rat
포장
antibody small pack of 25 μL
농도
~1 mg/mL
기술
immunoblotting: 1:125-1:250 using different cells extract
UniProt 수납 번호
배송 상태
dry ice
저장 온도
−20°C
타겟 번역 후 변형
unmodified
유전자 정보
human ... HSP90AA1(3320)
mouse ... HSP90AA1(15519)
rat ... HSP90AA1(299331)
일반 설명
Heat shock proteins 90 (HSP90) subunits α and β, also known as HSP90AA1 and HSP90AB1 respectively, belong to the heat shock proteins (HSPC) family of highly conserved chaperones proteins, which are classified according to their cellular localization and their expression pattern. These stress inducible proteins, which includes Hsp20, Hsp60, Hsp70, and Hsp90 are molecular chaperones that bind other proteins assisting their correct folding. Hsp90 is dispensable in bacteria but is essential, conserved and highly abundant in eukaryotes, even under nonstress conditions.
특이성
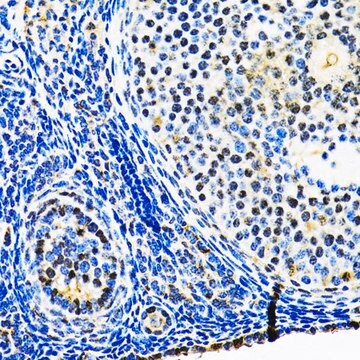
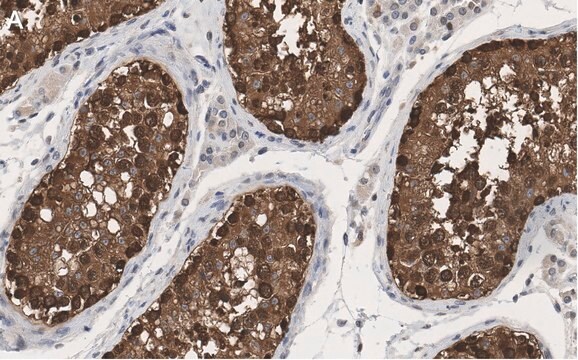
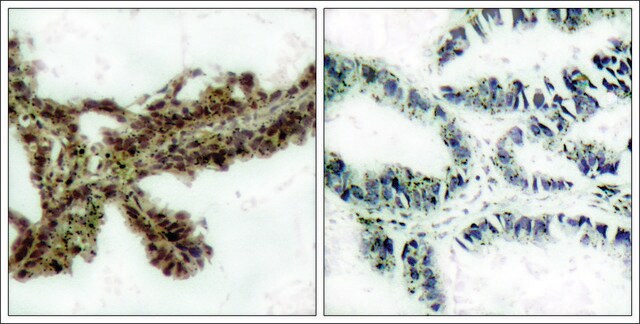
Anti-HSP90 antibody specifically recognizes human, mouse and rat HSP90.
면역원
Synthetic peptide corresponding to the C-terminal region of human HSP90, conjugated to KLH
애플리케이션
Anti-HSP90 antibody produced in rabbit has been used in various immunochemical techniques including immunoblotting (~90 kDa). Detection of the HSP90 band by Immunoblotting is specifically inhibited by the immunogen.
생화학적/생리학적 작용
The heat shock proteins 90 (Hsp90) proteins facilitates various essential physiological processes such as cell survival, cell cycle control, hormone signaling, and apoptosis. Upregulated expression of Hsp90 is associated with the development of various pathological conditions, including several types of cancer, viral infection, inflammation, and neurodegenerative diseases.
물리적 형태
Solution in 0.01 M phosphate buffered saline pH 7.4, containing 15 mM sodium azide as a preservative.
저장 및 안정성
For continuous use, store at 2-8°C for up to one month. For extended storage, freeze in working aliquots. Repeated freezing and thawing is not recommended. If slight turbidity occurs upon prolonged storage, clarify the solution by centrifugation before use. Working dilution samples should be discarded if not used within 12 hours.
면책조항
Unless otherwise stated in our catalog our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Faruk Tas et al.
Asian Pacific journal of cancer prevention : APJCP, 18(3), 599-601 (2017-04-26)
Background: Cellular heat shock proteins (HSPs) play significant roles in sustaining normal cellular conditions. The stimulated expressions of HSPs result in cellular stabilization at times of stress, such as cancer. The objective of this study was to determine the clinical
Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity
Park CJ, et al.
The plant pathology journal, 31, 323-323 (2015)
The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease
Hoter A, et al.
International Journal of Molecular Sciences, 19, 9-9 (2018)
Luke Whitesell et al.
Nature reviews. Cancer, 5(10), 761-772 (2005-09-22)
Standing watch over the proteome, molecular chaperones are an ancient and evolutionarily conserved class of proteins that guide the normal folding, intracellular disposition and proteolytic turnover of many of the key regulators of cell growth, differentiation and survival. This essential
Vanessa Strings-Ufombah et al.
Molecular therapy. Nucleic acids, 24, 67-78 (2021-03-20)
Oculopharyngeal muscular dystrophy (OPMD) is a rare autosomal dominant disease that results from an alanine expansion in the N-terminal domain of Poly-A Binding Protein Nuclear-1 (PABPN1). We have recently demonstrated that a two-vector gene therapy strategy significantly ameliorated the pathology
Global Trade Item Number
| SKU | GTIN |
|---|---|
| SAB4200812-100UL | |
| SAB4200812-25UL |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.