PZ0362
CP-640186 hydrochloride
≥95% (HPLC)
동의어(들):
(3R)-Anthracen-9-yl-[3-(morpholine-4-carbonyl)-[1,4′]bipiperidinyl-1′-yl]-methanone hydrochloride, CP 640186 hydrochloride, CP640186 hydrochloride, [(3R)-1′-(9-Anthracenylcarbonyl)[1,4′-bipiperidin]-3-yl]-4-morpholinyl-methanone hydrochloride
About This Item
추천 제품
Quality Level
분석
≥95% (HPLC)
양식
powder
저장 조건
desiccated
색상
white to beige
solubility
H2O: 2 mg/mL, clear (warmed)
저장 온도
room temp
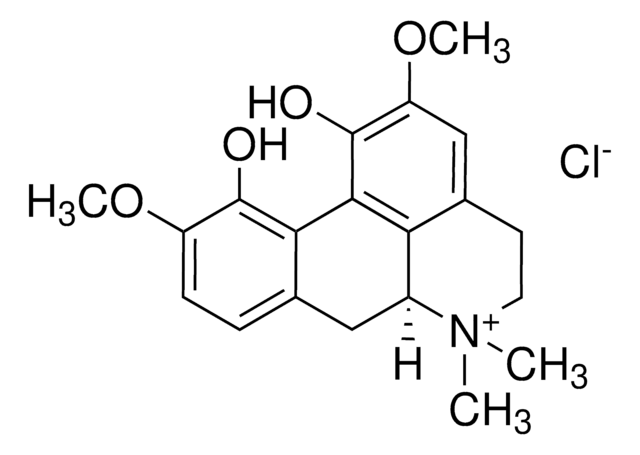
SMILES string
O=C(C1=C2C=CC=CC2=CC3=CC=CC=C31)N(CC4)CCC4N5C[C@H](C(N6CCOCC6)=O)CCC5.[H]Cl
InChI
1S/C30H35N3O3.ClH/c34-29(32-16-18-36-19-17-32)24-8-5-13-33(21-24)25-11-14-31(15-12-25)30(35)28-26-9-3-1-6-22(26)20-23-7-2-4-10-27(23)28;/h1-4,6-7,9-10,20,24-25H,5,8,11-19,21H2;1H/t24-;/m1./s1
InChI key
DUBNXJIOBFRASV-GJFSDDNBSA-N
애플리케이션
생화학적/생리학적 작용
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.