추천 제품
생물학적 소스
human female liver (hepatocarcinoma and hepatitis C tumor)
Quality Level
형태
liquid
OMIM 수납 번호
저장 온도
−196°C
유전자 정보
human ... OATP1B3(28234)
일반 설명
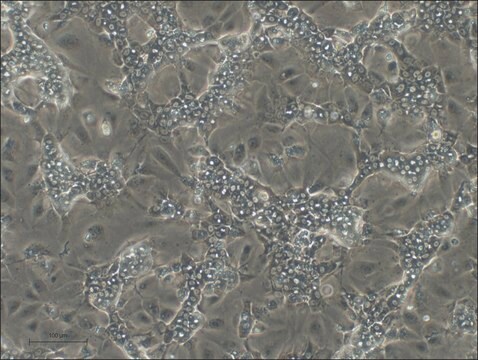
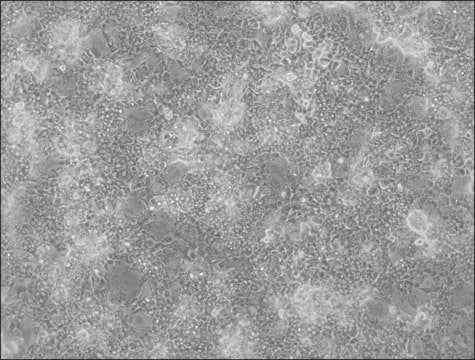
HepaRG is a human hepatoma cell line. The cells possess a pseudodiploid karyotype and have been characterized as an oval ductular bipotent hepatic cell line as they have the ability to differentiate into both biliary and hepatocyte lineages in the presence of DMSO. HepaRG OATP1B3 knockout cells express the major xenobiotic sensors (PXR, CAR and AhR), drug transporters, phase I and II drug metabolizing enzymes as well as key hepatic transcription factors involved in stress response pathways.
애플리케이션
See technical bulletin for detailed protocols
특징 및 장점
Zinc finger nucleases (ZFN) mediated Knock-out of SLCO1B3 (MRP3) gene.
- The frame-shift mutation of SLCO1B3 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
품질
Tested for Mycoplasma, sterility, post-freeze viability, short terminal repeat (STR) analysis for cell line identification, cytochrome oxidase I (COI) analysis for cell line species confirmation.
법적 정보
These products are covered by the License Agreement as described in Exhibit 1, 2 and 3, in the technical bulletin.
HepaRG is a trademark of BioPredic International company
면책조항
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
문서
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.